KTGRA02FF3
Aervent® 0.2 µm, Opticap® XL Capsule
Opticap® XL 2, inlet connection diam. 3/4 in., pore size 0.2 μm, cartridge nominal length 5.3 in. (13.5 cm)
Synonym(s):
Opticap Sterilizing Grade XL2 Aervent 0.2 µm 3/4 in. TC/TC
About This Item
Recommended Products
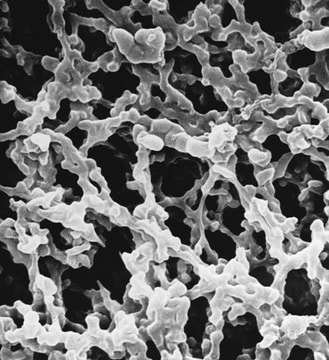
material
PTFE
polypropylene
polypropylene housing
polypropylene support
polypropylene vent cap
silicone seal
Quality Level
sterility
non-sterile
sterilization compatibility
autoclavable compatible
product line
Opticap® XL 2
feature
hydrophobic
manufacturer/tradename
Opticap®
parameter
1.0 bar max. differential pressure (15 psid) at 80 °C (Forward)
1.0 bar max. inlet pressure (15 psi) at 80 °C
2.8 bar max. inlet pressure (40 psi) at 60 °C
25 °C max. inlet temp.
4.1 bar max. differential pressure (60 psid) at 25 °C (Reverse; intermittent)
5.5 bar max. differential pressure (80 psid) at 25 °C (Forward)
5.5 bar max. inlet pressure (80 psi) at 23 °C
80 psig max. inlet pressure
technique(s)
gas filtration: suitable
L
5.6 in.
W
3.3 in.
cartridge nominal length
5.3 in. (13.5 cm)
diam.
8.4 cm (3.3 in.)
filtration area
0.1 m2
inlet connection diam.
3/4 in.
inlet to outlet W
13.5 cm (5.3 in.)
outlet connection diam.
3/4 in.
impurities
<0.5 EU/mL USP bacterial endotoxins (LAL test, sample aqueous extraction)
gravimetric extractables
≤15 mg
matrix
Aervent®
pore size
0.2 μm pore size
bubble point
≥1100 mbar (16 psig), nitrogen with 70/30% IPA/water at 23 °C
fitting
1/4 in. drain/vent hose barb (with double O-ring Seal)
inlet sanitary flange
outlet sanitary flange
(19 mm (3/4 in.) Sanitary Flange Inlet and Outlet)
Looking for similar products? Visit Product Comparison Guide
General description
Packaging
Linkage
Preparation Note
30 autoclave cycles of 30 min @ 135 °C; not in-line steam sterilizable
Analysis Note
Samples were quantitatively retentive of a minimum Brevundimonas diminuta challenge concentration of 1 x 10⁷ CFU/cm² using ASTM® F838 methodology.
Other Notes
- Organism Retention: Microorganism
- Mode of Action: Filtration (size exclusion)
- Application: BioProcessing
- Intended Use: Reduction or removal of microorganism/bioburden
- Instructions for Use: Please refer Steam Sterilization & Integrity Testing Procedures guide and first page of Visual Inspection Guide
- Storage Statement: Store in dry location
- Disposal Statement: Dispose of in accordance with applicable federal, state and local regulations.
Legal Information
Disclaimer
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service