ABS30
Anti-Sulfenic Acid Modified Cysteine (2-Thiodimedone-Specific Ig) Antibody
serum, from rabbit
Synonym(s):
Sulfenic Acid Modified Cysteine (2-Thiodimedone-Specific Ig)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352203
eCl@ss:
32160702
NACRES:
NA.41
Recommended Products
biological source
rabbit
Quality Level
antibody form
serum
antibody product type
primary antibodies
clone
polyclonal
species reactivity
human, mouse, rat
species reactivity (predicted by homology)
all
technique(s)
western blot: suitable
isotype
IgG
shipped in
wet ice
target post-translational modification
unmodified
General description
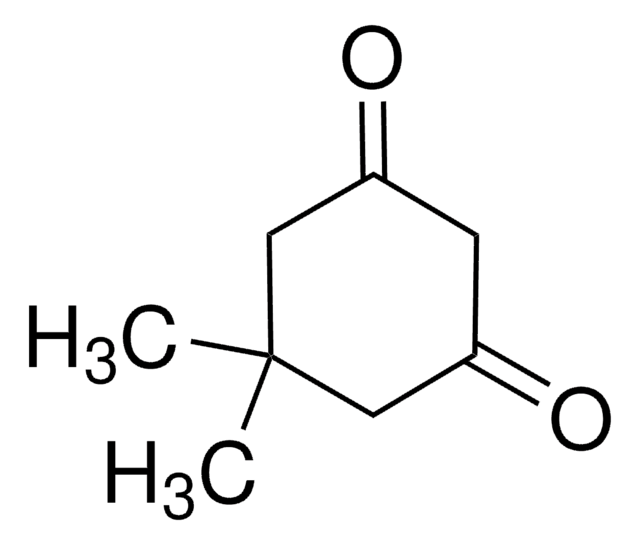
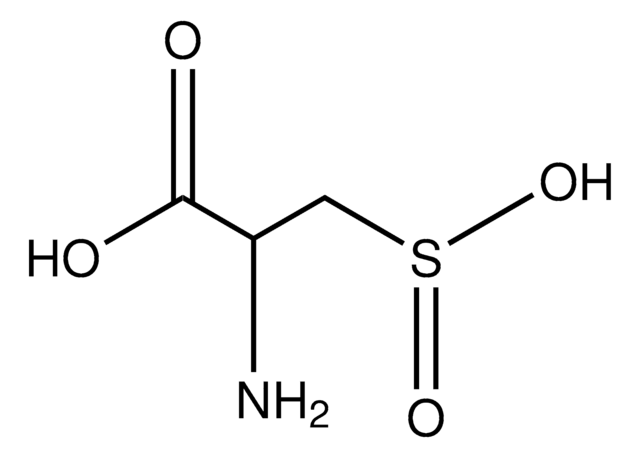
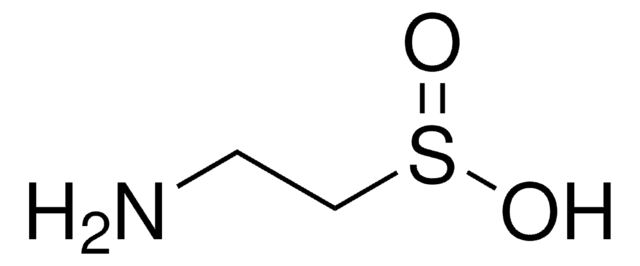
Protein sulfenic acid formation is a reversible post-translational modification that may be used to monitor protein oxidation on reactive cysteines within target proteins. This can be detected with protein sulfenic acid derivatised with dimedone.
Specificity
This anitbody recognizes sulfenic acid modified proteins. Pan modification against all species.
Immunogen
Linear peptide corresponding to sulfenic acid modified proteins.
Application
Anti-Sulfenic Acid Modified Cysteine (2-Thiodimedone-Specific Ig) Antibody detects level of Sulfenic Acid Modified Cysteine & has been published & validated for use in WB.
Immunofluorescence Analysis: A previous lot was used by an independent laboratory in IF. (Seo, YH, et al. (2009). PNAS. 106(38): 16163-16168.)
Quality
Evaluated by Western Blot in rat ventricular myocyte lysate.
Western Blot Analysis: 01:1,000 dilution of this antibody detected sulfenic acid modified proteins on 10 µg of rat ventricular myocyte lysate.
Western Blot Analysis: 01:1,000 dilution of this antibody detected sulfenic acid modified proteins on 10 µg of rat ventricular myocyte lysate.
Target description
Pan antibody smear is expected.
Not finding the right product?
Try our Product Selector Tool.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ester Zito et al.
Molecular cell, 48(1), 39-51 (2012-09-18)
Endoplasmic reticulum (ER) thiol oxidases initiate a disulfide relay to oxidatively fold secreted proteins. We found that combined loss-of-function mutations in genes encoding the ER thiol oxidases ERO1α, ERO1β, and PRDX4 compromised the extracellular matrix in mice and interfered with
Juliana Navarro-Yepes et al.
Molecular neurobiology, 53(8), 5229-5251 (2015-09-28)
Intracytoplasmic inclusions of protein aggregates in dopaminergic cells (Lewy bodies) are the pathological hallmark of Parkinson's disease (PD). Ubiquitin (Ub), alpha (α)-synuclein, p62/sequestosome 1, and oxidized proteins are the major components of Lewy bodies. However, the mechanisms involved in the
Angel Stanoev et al.
Cell systems, 7(3), 295-309 (2018-08-27)
The proto-oncogenic epidermal growth factor receptor (EGFR) is a tyrosine kinase whose sensitivity to growth factors and signal duration determines cellular behavior. We resolve how EGFR's response to epidermal growth factor (EGF) originates from dynamically established recursive interactions with spatially
Lianggong Ding et al.
Nature metabolism, 3(12), 1648-1661 (2021-12-15)
To liberate fatty acids (FAs) from intracellular stores, lipolysis is regulated by the activity of the lipases adipose triglyceride lipase (ATGL), hormone-sensitive lipase and monoacylglycerol lipase. Excessive FA release as a result of uncontrolled lipolysis results in lipotoxicity, which can
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service