375680
Hesperadin
Hesperadin primarily used in Inhibition.
Synonym(s):
Hesperadin, Mammalian Ste20-like Kinase 4 Inhibitor, MST4 Inhibitor, Aurora Kinase Inhibitor X, AMPK Inhibitor II
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
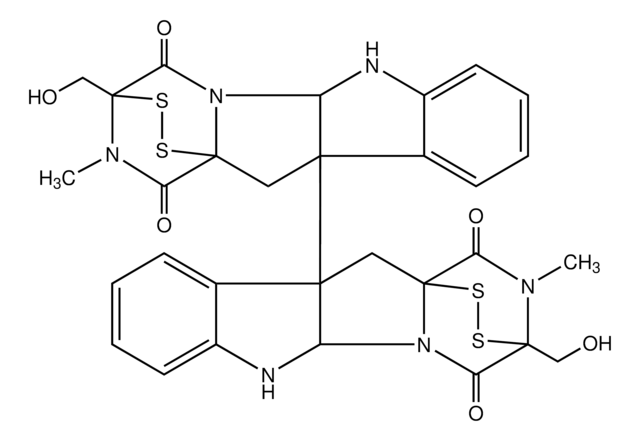
C29H32N4O3S
CAS Number:
Molecular Weight:
516.65
UNSPSC Code:
12352200
Recommended Products
Quality Level
assay
≥95% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
yellow
solubility
DMSO: 50 mg/mL
shipped in
ambient
storage temp.
−20°C
General description
A cell-permeable indolinone compound that interacts with both the ATP- and the adjacent hydrophobic binding pocket of Aurora B and acts as a rapid and reversible inhibitor of Aurora kinase B activity (IC50 ~ 250 nM) with moderate selectivity over Cdk1/B, Cdk2/E and Cdk4/D1 (IC50 = 1.2, >10 and >10 µM). Also, potently inhibits several kinases at 1 µM in a 25-kinase screening panel (100%, 100%, 96%, 88%, 87% and 84% against Lck, PHK, AMPK, MKK1, MAPKAP-K1 and Chk1, respectively). Reported to reduce histone H3-Ser10 phosphorylation, inhibit cytokinesis, promote chromosome biorientation, perturb mitosis and polyploidy in mammalian cells. Recently shown to potently inhibit MST4 kinase (IC50 = 6.18 nM) in an ATP-competitive manner. Dose-dependently accelerates hypoxia-induced apoptosis and decreases survival (EC50 = 10 nM in MST4 LβT2 gonadotrope cells).
A cell-permeable indolinone compound that interacts with both the ATP- and the adjacent hydrophobic binding pocket of Aurora B and acts as a rapid and reversible inhibitor of Aurora kinase B activity (IC50 ~250 nM) with moderate selectivity over Cdk1/B, Cdk2/E and Cdk4/D1 (IC50 = 1.2, >10 and >10 µM). Also, potently inhibits several kinases at 1 µM in a 25-kinase screening panel (100%, 100%, 96%, 88%, 87% and 84% against Lck, PHK, AMPK, MKK1, MAPKAP-K1 and Chk1, respectively). Reported to reduce histone H3-Ser10 phosphorylation, inhibit cytokinesis, promote chromosome biorientation, perturb mitosis and polyploidy in mammalian cells.
Packaging
Packaged under inert gas
Warning
Toxicity: Regulatory Review (Z)
Reconstitution
Following reconstitution, aliquot freeze at -(20°C). Stock solutions are stable for up to 6 months at -20°C..
Other Notes
Xiong, W., et al. 2016. Mol. Cancer Ther.15, In press.
Knowlton, A.L., et al. 2006. Curr. Biol.16, 1705.
Kapoor, T.M., et al. 2006. Science311, 388.
Hirota, T., et al. 2005. Nature438, 1176.
Sessa, F., et al. 2005. Mol. Cell.18, 379.
Lampson, M.A., et al. 2004. Nat. Cell Biol.6, 232.
Hauf, S., et al. 2003. J. Cell Biol.161, 281.
Knowlton, A.L., et al. 2006. Curr. Biol.16, 1705.
Kapoor, T.M., et al. 2006. Science311, 388.
Hirota, T., et al. 2005. Nature438, 1176.
Sessa, F., et al. 2005. Mol. Cell.18, 379.
Lampson, M.A., et al. 2004. Nat. Cell Biol.6, 232.
Hauf, S., et al. 2003. J. Cell Biol.161, 281.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service