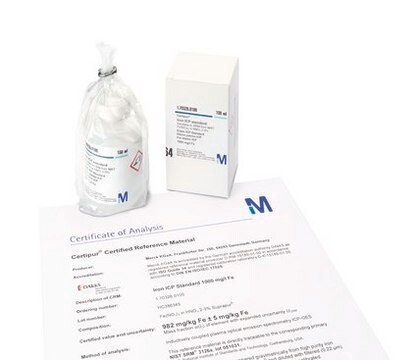
1.70313
Cobalt ICP standard
traceable to SRM from NIST Co(NO₃)₂ in HNO₃ 2-3% 1000 mg/l Co Certipur®
Synonym(s):
Nota 1 A
About This Item
Recommended Products
grade
certified reference material
Quality Level
agency
suitable for EPA 200.7
suitable for EPA 200.8
product line
Certipur®
technique(s)
ICP: suitable
pH
0.5 (20 °C in H2O)
density
1.014 g/cm3 at 20 °C
application(s)
industrial qc
pharmaceutical
format
single component solution
storage temp.
15-25°C
General description
Please visit ISO certificates and Site Quality Self-Assessments to access the current certificates of accreditation.
Download your certificate at http://www.sigma-aldrich.com to view certified values, including uncertainty, date of expiry, and detailed information about trace impurities.
Application
- Medical device monitoring: A method using ICP-MS was developed and validated for determining cobalt in patients with metal-on-metal prostheses, illustrating the importance of cobalt ICP standards in clinical surveillance and patient safety (Capiau et al., 2020).
- Pharmaceutical quality control: Cobalt ICP standards are essential for ensuring the purity and compliance of pharmaceutical products, as evidenced by their application in the quality control processes within the pharmaceutical industry (Knoop et al., 2020).
Other Notes
Legal Information
signalword
Danger
Hazard Classifications
Aquatic Chronic 3 - Carc. 1B - Eye Irrit. 2 - Met. Corr. 1 - Repr. 1B - Skin Irrit. 2
Storage Class
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
Atomic spectroscopy uses the energy absorbed or emitted by electrons to identify and quantify the elemental composition of a sample. It includes various analytical techniques, such as AAS, AES, FAA, GFAA, ICP-OES, ICP-MS and XRF.
Atomic spectroscopy uses the energy absorbed or emitted by electrons to identify and quantify the elemental composition of a sample. It includes various analytical techniques, such as AAS, AES, FAA, GFAA, ICP-OES, ICP-MS and XRF.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service