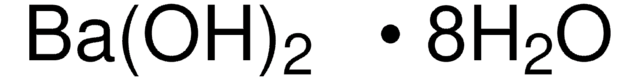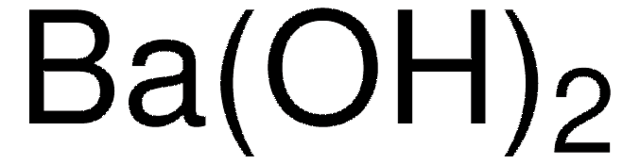1.01735
Barium hydroxide octahydrate
EMPLURA®
Synonym(s):
Barium hydroxide octahydrate, Caustic baryta, Barium oxide hydrate octahydrate
About This Item
Recommended Products
Quality Level
product line
EMPLURA®
assay
≥98.0% (acidimetric)
form
solid
potency
550 mg/kg LD50, oral (Rat)
impurities
≤0.01% Substances insoluble in dilute hydrochloric acid
≤0.2% Substances not precipitated by dilute sulfuric acid (as sulfate)
pH
14 (20 °C in H2O, saturated aqueous solution)
mp
78 °C
solubility
72 g/L
density
2.18 g/cm3 at 20 °C
bulk density
900‑1100 kg/m3
anion traces
carbonate (as BaCO3): ≤2.0%
chloride (Cl-): ≤0.002%
sulfide (S2-): ≤0.001%
cation traces
Ca: ≤0.005%
Fe: ≤0.001%
Sr: ≤1.0%
heavy metals (as Pb): ≤0.002%
storage temp.
2-30°C
InChI
1S/Ba.10H2O/h;10*1H2/q+2;;;;;;;;;;/p-2
InChI key
ZUDYPQRUOYEARG-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
- Chitosan and carbon nitride doped barium hydroxide nanoparticles served as dye degrader and bactericidal potential: A molecular docking study.: This study explores the use of barium hydroxide octahydrate nanoparticles doped with chitosan and carbon nitride for applications in dye degradation and as bactericidal agents. The research highlights the potential of these nanoparticles in environmental remediation and antimicrobial treatments (Ikram et al., 2023).
- Ba(OH)2 Equilibria in the System Ba-O-H-F, With Application to the Formation of Ba2YCu3O6.5 + x From BaF2-Precursors.: This paper discusses the equilibrium of barium hydroxide octahydrate in the Ba-O-H-F system and its application in forming Ba2YCu3O6.5 + x from BaF2 precursors. This research is relevant to material synthesis and applications in superconductors (Cook et al., 2005).
- The Effect of Nanometer Oxide Additive on the Heat-Conducting Property of Barium Hydroxide Octahydrate.: This article investigates how nanometer-scale oxide additives affect the heat-conducting properties of barium hydroxide octahydrate, highlighting potential applications in thermal management systems and heat storage (Xu et al., 2015).
- Application of capillary isotachophoresis to analysis of serum protein.: This study utilizes barium hydroxide octahydrate in the capillary isotachophoresis analysis of serum proteins, demonstrating its application in biochemical analysis and clinical diagnostics (Ono et al., 1990).
- Staining of tissue sections for electron microscopy with heavy metals. II. Application of solutions containing lead and barium.: This research applies barium hydroxide octahydrate solutions in staining tissue sections for electron microscopy, providing insights into its use in histological and cytological studies (WATSON, 1958).
Analysis Note
Substances insoluble in dilute hydrochloric acid: ≤ 0.01 %
Carbonate (as BaCO₃): ≤ 2.0 %
Chloride (Cl): ≤ 0.002 %
Sulphide (S): ≤ 0.001 %
Heavy metals (as Pb): ≤ 0.002 %
Ca (Calcium): ≤ 0.005 %
Fe (Iron): ≤ 0.001 %
Sr (Strontium): ≤ 1.0 %
Substances not precipitated by dilute sulfuric acid (as sulfate): ≤ 0.2 %
Legal Information
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class
6.1A - Combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service