1.00631
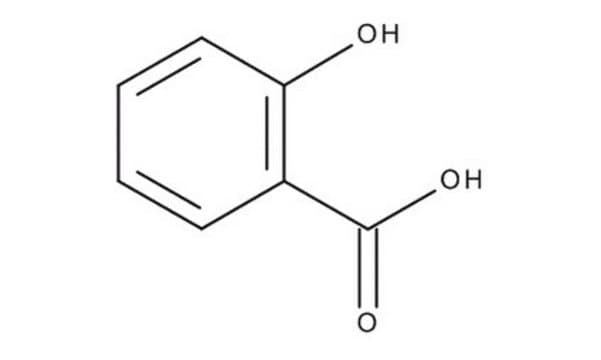
Salicylic acid
EMPROVE® ESSENTIAL Ph Eur,BP,USP
Pharma Manufacturing
Synonym(s):
Salicylic acid, 2-Hydroxybenzoic acid
About This Item
Recommended Products
agency
BP
Ph. Eur.
USP
Quality Level
vapor pressure
<1 hPa ( 100 °C)
product line
EMPROVE® ESSENTIAL
assay
98.0-102.0% dry basis (HPLC)
form
solid
autoignition temp.
500 °C
potency
1250-1580 mg/kg LD50, oral (Rat)
>2000 mg/kg LD50, skin (Rat)
technique(s)
API processing | cocrystal formation: suitable
API processing | salt formation: suitable
pH
2.4 ( in H2O, saturated solution)
bp
211 °C/1013 hPa
mp
157-159 °C
transition temp
flash point 157 °C
solubility
2 g/L
density
1.443 g/cm3 at 20 °C
bulk density
400‑500 kg/m3
anion traces
chloride (Cl-): ≤0.0100%
sulfate (SO42-): ≤0.0200%
application(s)
liquid formulation
pharmaceutical
storage temp.
15-25°C
SMILES string
[O-]c1c(cccc1)C(=O)O.[H+]
InChI
1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10)
InChI key
YGSDEFSMJLZEOE-UHFFFAOYSA-N
General description
Application
Legal Information
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Repr. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
314.6 °F - closed cup
flash_point_c
157 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Summary application report for analysis of moisture in Ammonia, aqueous
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service