W288608
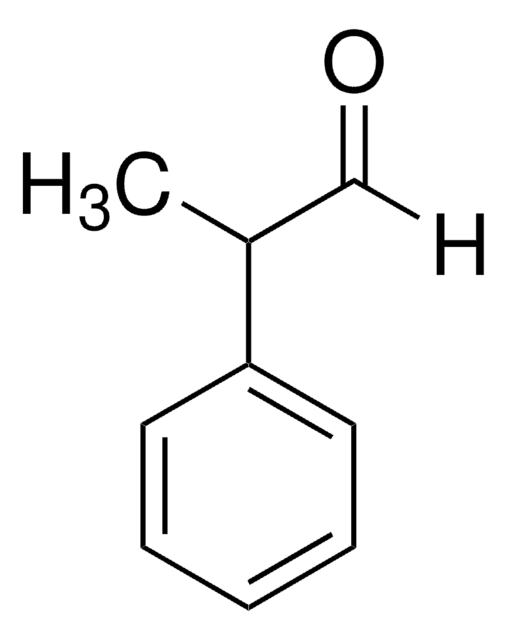
2-Phenylpropionaldehyde
≥95%, FCC, FG
Synonym(s):
2-Phenylpropanal, Hydratropaldehyde
About This Item
Halal
Kosher
Recommended Products
biological source
synthetic
Quality Level
grade
FG
Halal
Kosher
agency
meets purity specifications of JECFA
reg. compliance
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 172.515
assay
≥95%
refractive index
n20/D 1.517 (lit.)
bp
92-94 °C/12 mmHg (lit.)
density
1.002 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
organoleptic
fresh; green; floral
storage temp.
2-8°C
SMILES string
[H]C(=O)C(C)c1ccccc1
InChI
1S/C9H10O/c1-8(7-10)9-5-3-2-4-6-9/h2-8H,1H3
InChI key
IQVAERDLDAZARL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Cytotoxicity, early safety screening, and antimicrobial potential of minor oxime constituents of essential oils and aromatic extracts.: Explores the safety and effectiveness of 2-Phenylpropionaldehyde among other compounds in essential oils, highlighting its potential antimicrobial properties and implications for food safety and preservation (Strub DJ et al., 2022).
- Spectroscopic Evidence for a Cobalt-Bound Peroxyhemiacetal Intermediate.: This study provides spectroscopic evidence of a cobalt-bound intermediate in reactions involving 2-Phenylpropionaldehyde, advancing our knowledge of chemical reaction mechanisms and catalysis (Cho J et al., 2021).
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
174.2 °F
flash_point_c
79 °C
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W288608-10KG | |
| W288608-1KG | |
| W288608-5KG | |
| W288608-SAMPLE-K | 4061837517570 |
| W288608-10KG-K | 4061837835254 |
| W288608-1KG-K | 4061837835261 |
| W288608-5KG-K | 4061837835278 |
| W288608-SAMPLE |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service