All Photos(1)
About This Item
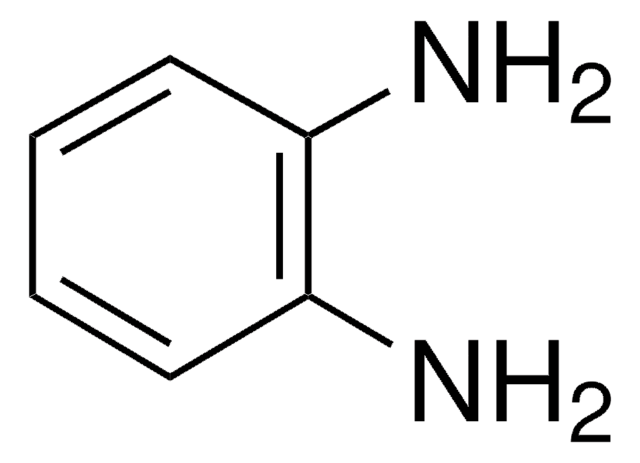
Empirical Formula (Hill Notation):
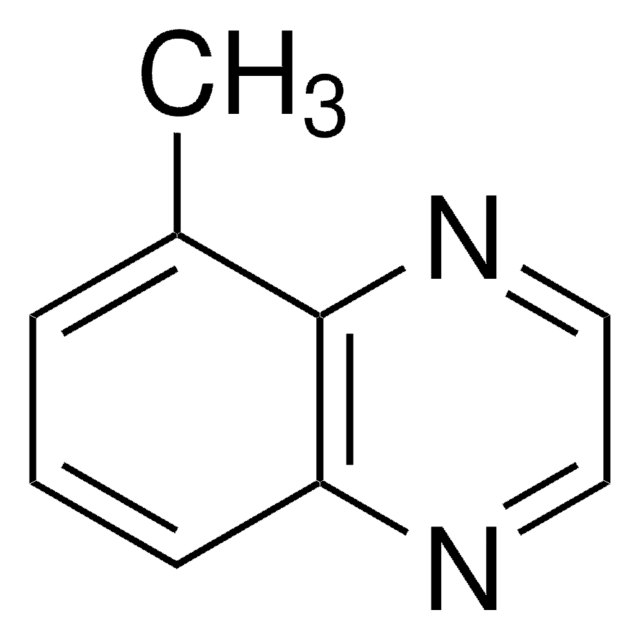
C9H8N2
CAS Number:
Molecular Weight:
144.17
Beilstein/REAXYS Number:
113307
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
liquid
refractive index
n20/D 1.613 (lit.)
bp
245-247 °C (lit.)
density
1.118 g/mL at 25 °C (lit.)
SMILES string
Cc1cnc2ccccc2n1
InChI
1S/C9H8N2/c1-7-6-10-8-4-2-3-5-9(8)11-7/h2-6H,1H3
InChI key
ALHUXMDEZNLFTA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
224.6 °F - closed cup
flash_point_c
107 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
F W Chaplen et al.
Proceedings of the National Academy of Sciences of the United States of America, 95(10), 5533-5538 (1998-05-20)
Methylglyoxal is an alpha-ketoaldehyde and dicarbonyl formed in cells as a side product of normal metabolism. Endogenously produced dicarbonyls, such as methylglyoxal, are involved in numerous pathogenic processes in vivo, including carcinogenesis and advanced glycation end-product formation; advanced glycation end-products
Determination of methylglyoxal as 2-methylquinoxaline by high-performance liquid chromatography and its application to biological samples.
S Ohmori et al.
Journal of chromatography, 414(1), 149-155 (1987-02-20)
Methylglyoxal assay in cells as 2-methylquinoxaline using 1,2-diaminobenzene as derivatizing reagent.
C Cordeiro et al.
Analytical biochemistry, 234(2), 221-224 (1996-02-15)
Umashankar Das et al.
Bioorganic & medicinal chemistry, 17(11), 3909-3915 (2009-05-12)
A series of 2-(3-aryl-2-propenoyl)-3-methylquinoxaline-1,4-dioxides 3a-l were prepared by condensation of various aryl aldehydes with 2-acetyl-3-methylquinoxaline-1,4-dioxide 2. These compounds inhibit the growth of human Molt 4/C8 and CEM T-lymphocytes and the IC(50) values are mainly in the 5-30 microM range. The
Weerachat Sompong et al.
BMC complementary and alternative medicine, 15, 394-394 (2015-11-02)
Methylglyoxal (MG) is one of the most reactive glycating agents, which result the formation of advanced glycation end-products (AGEs) that have been implicated in the progression of age-related diseases. Inhibition of MG-induced AGE formation is the imperative approach for alleviating
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service