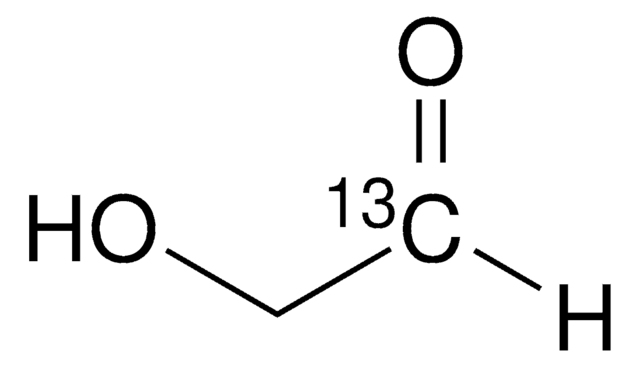
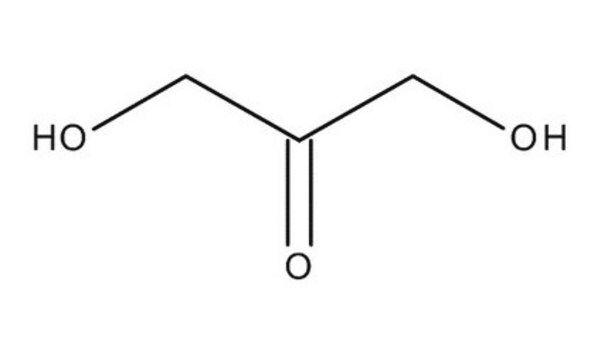
G6805
Glycolaldehyde dimer
crystalline, mixture of stereoisomers. Melts between 80 and 90 °C depending on stereoisomeric composition
Synonym(s):
1,4-Dioxane-2,5-diol, 2,5-Dihydroxy-1,4-dioxane, Hydroxyacetaldehyde dimer
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O4
CAS Number:
Molecular Weight:
120.10
Beilstein/REAXYS Number:
506029
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
form
crystalline
storage temp.
2-8°C
SMILES string
OC1COC(O)CO1
InChI
1S/C4H8O4/c5-3-1-7-4(6)2-8-3/h3-6H,1-2H2
InChI key
ATFVTAOSZBVGHC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Glycolaldehyde dimer may be used in the synthesis of 3,4-diaza-2-hexene-1,6-diol, which can undergo hydrogenation to form 1,2-bis(2-hydroxyethyl)hydrazine. It undergoes cycloaddition with 2,3-dihydrofuran in the presence of a chiral catalyst to form fused bicyclic tetrahydrofuran (bis-THF) alcohol, a key moiety of HIV protease inhibitors.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
1, 2-bis (2-hydroxyethyl) hydrazine and derivatives.
Nielsen AT
The Journal of Organic Chemistry, 42(17), 2900-2902 (1977)
Hiromi Niwa et al.
Journal of natural medicines, 75(1), 194-200 (2020-09-26)
The production and accumulation of advanced glycation end products (AGEs) have been implicated in diabetes and diabetic complication. This study was conducted as a search for an AGE inhibitor from brown alga, Sargassum macrocarpum. Separation and purification were performed using
Efficient Synthesis of (3 R, 3a S, 6a R)-Hexahydrofuro [2, 3-b] furan-3-ol from Glycolaldehyde.
Canoy WL
Organic Letters, 10(6), 1103-1106 (2008)
Stéphan Houdier et al.
Analytical and bioanalytical chemistry, 410(27), 7031-7042 (2018-08-11)
Derivatization techniques based on α-effect amines and H+ catalysis are commonly used for the measurement of carbonyl compounds (CCs), whether in environmental, food, or biological samples. Here, we investigated the potential of aniline-based catalysts to improve derivatization rates of selected
Qiying Liu et al.
ChemSusChem, 12(17), 3977-3987 (2019-06-22)
Ethanol is an important bulk chemical with diverse applications. Biomass-derived ethanol is traditionally produced by fermentation. Direct cellulose conversion to ethanol by chemocatalysis is particularly promising but remains a great challenge. Herein, a one-pot hydrogenolysis of cellulose into ethanol was
Articles
Noble-Metal Nanostructures with Controlled Morphologies
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service