F20408
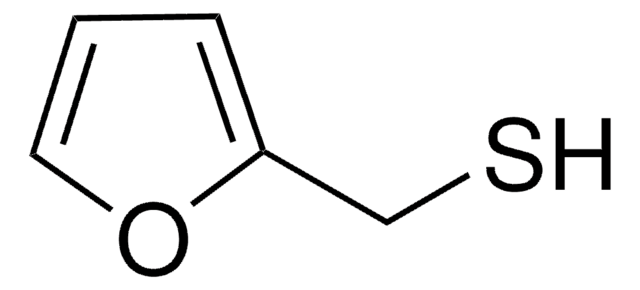
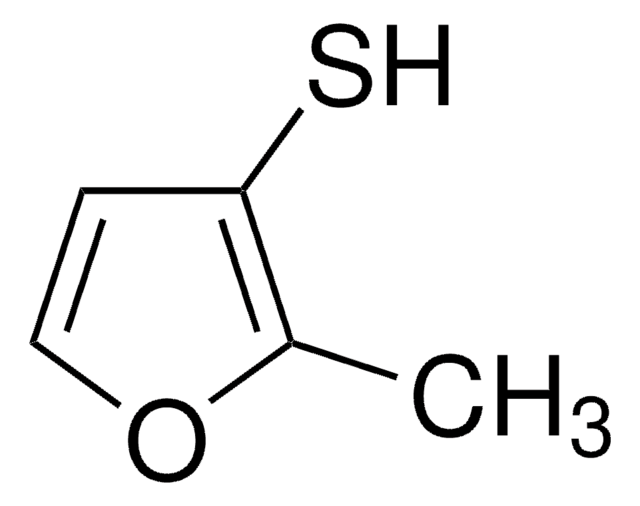
2-Furanmethanethiol
98%
Synonym(s):
2-Furfurylthiol, 2-Furylmethanethiol, Furfuryl mercaptan
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H6OS
CAS Number:
Molecular Weight:
114.17
Beilstein/REAXYS Number:
383594
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
liquid
refractive index
n20/D 1.531 (lit.)
bp
155 °C (lit.)
density
1.132 g/mL at 25 °C (lit.)
SMILES string
SCc1ccco1
InChI
1S/C5H6OS/c7-4-5-2-1-3-6-5/h1-3,7H,4H2
InChI key
ZFFTZDQKIXPDAF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christoph Müller et al.
Journal of agricultural and food chemistry, 54(26), 10076-10085 (2006-12-21)
Recent investigations demonstrated that the reaction of odor-active thiols such as 2-furfurylthiol with thermally generated chlorogenic acid degradation products is responsible for the rapid aroma staling of coffee beverages. To get a clear understanding of the molecular mechanisms underlying this
Christoph Müller et al.
Journal of agricultural and food chemistry, 55(10), 4095-4102 (2007-04-19)
To gain a more comprehensive knowledge of the contribution of recently identified phenol/thiol conjugates to the storage-induced degradation of odorous thiols, the concentrations of the sulfury-roasty smelling key odorant 2-furfurylthiol and the concentrations of the putative thiol-receptive di- and trihydroxybenzenes
Christoph Müller et al.
Journal of agricultural and food chemistry, 53(7), 2623-2629 (2005-03-31)
The purpose of the following study was to investigate the influence of coffee roasting on the thiol-binding activity of coffee beverages, and to investigate the potential of various green bean compounds as precursors of thiol-binding sites by using promising "in
Brian G Lake et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 41(12), 1761-1770 (2003-10-18)
The metabolism of two thiofurans, namely furfuryl mercaptan (FM) and 2-methyl-3-furanthiol (MTF), to their corresponding methyl sulphide and methyl sulphoxide derivatives has been studied in male Sprague-Dawley rat hepatocytes and liver microsomes. Rat hepatocytes converted FM to furfuryl methyl sulphoxide
Luigi Poisson et al.
Journal of agricultural and food chemistry, 57(21), 9923-9931 (2009-10-13)
The formation of several key odorants, such as 2-furfurylthiol (FFT), alkylpyrazines, and diketones, was studied upon coffee roasting. The approach involved the incorporation of potential precursors in green coffee beans by means of biomimetic in-bean and spiking experiments. Both labeled
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service