D108405
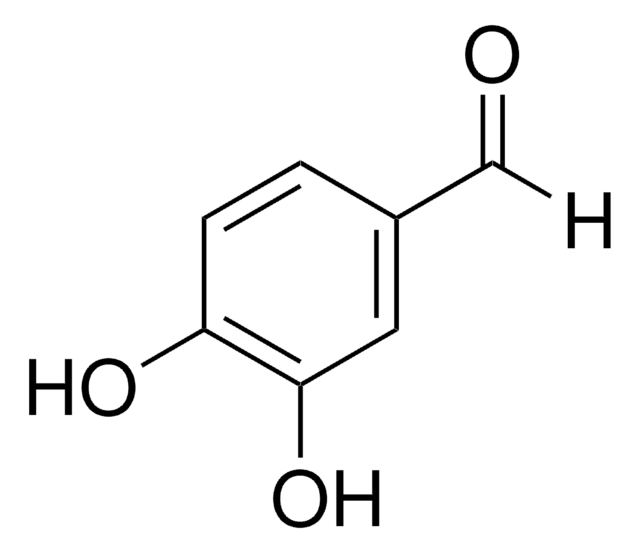
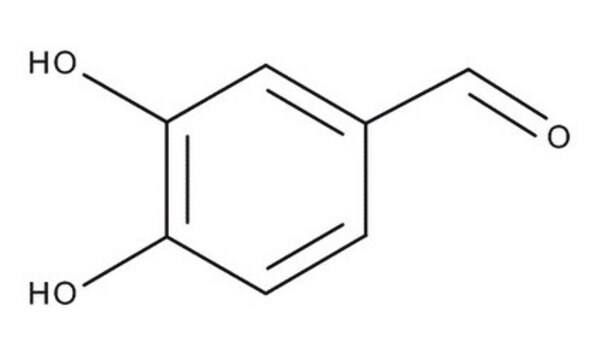
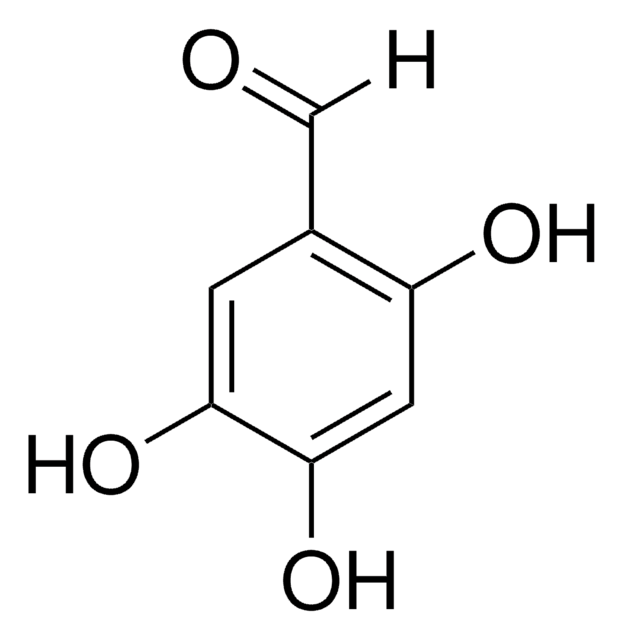
3,4-Dihydroxybenzaldehyde
97%
Synonym(s):
Protocatechualdehyde
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
(HO)2C6H3CHO
CAS Number:
Molecular Weight:
138.12
Beilstein/REAXYS Number:
774381
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
powder
mp
150-157 °C (lit.)
SMILES string
Oc1ccc(C=O)cc1O
InChI
1S/C7H6O3/c8-4-5-1-2-6(9)7(10)3-5/h1-4,9-10H
InChI key
IBGBGRVKPALMCQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Dihydroxybenzaldehyde can be used as a reactant for the synthesis of:
- Copolymers containing poly(p-phenylenevinylene) chromophore to be used in light-emitting electrochemical cell.
- 2-Arylbenzothiazoles with potential application as anti-cancer agents against human colon cancer cells.
- Variety of thiazolidin-4-one ring systems having antimicrobial activity.
- Bis-Schiff bases of isatins which can be used as antiglycating agents.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of bis-Schiff bases of isatins and their antiglycation activity.
Khan KM, et al.
Bioorganic & Medicinal Chemistry, 17(22), 7795-7801 (2009)
Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials.
Kavitha CV, et al.
Bioorganic & Medicinal Chemistry, 14(7), 2290-2299 (2006)
Synthesis and electroluminescence of novel copolymers containing crown ether spacers.
Sun Q, et al.
Journal of Materials Chemistry, 13(4), 800-806 (2003)
Catriona G Mortimer et al.
Journal of medicinal chemistry, 49(1), 179-185 (2006-01-06)
A series of new 2-phenylbenzothiazoles has been synthesized on the basis of the discovery of the potent and selective in vitro antitumor properties of 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (8n; GW 610, NSC 721648). Synthesis of analogues substituted in the benzothiazole ring was achieved
Narsimha Mamidi et al.
The journal of physical chemistry. B, 116(35), 10684-10692 (2012-08-07)
Diacylglycerol (DAG) regulates a broad range of cellular functions including tumor promotion, apoptosis, differentiation, and growth. Thus, the DAG-responsive C1 domain of protein kinase C (PKC) isoenzymes is considered to be an attractive drug target for the treatment of cancer
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service