924253
Deoxazole-quat
≥95%
Synonym(s):
5,7-Di-tert-butyl-3-(4-(trifluoromethyl)phenyl)benzo[d]oxazol-3-ium tetrafluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C22H25BF7NO
CAS Number:
Molecular Weight:
463.24
MDL number:
UNSPSC Code:
12161600
NACRES:
NA.21
Recommended Products
Quality Level
assay
≥95%
form
powder
mp
230-234 °C
SMILES string
CC(C)(C)C1=CC([N+](C2=CC=C(C(F)(F)F)C=C2)=CO3)=C3C(C(C)(C)C)=C1.F[B-2](F)(F)F
Application
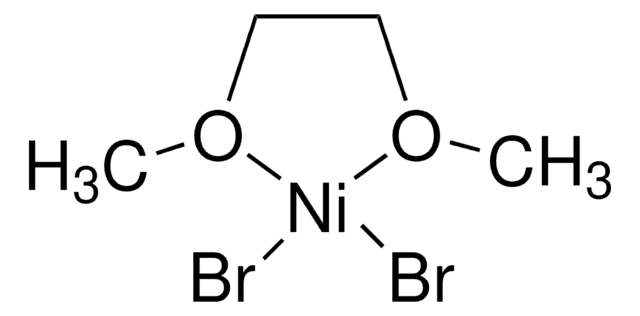
Deoxazole-quat is an N-heterocyclic carbene salt which activates tertiary free alcohols for a C-C cross coupling reaction with aryl halides. The MacMillan group developed a mild, robust, and selective metallaphotoredox-based cross-coupling platform for the deoxygenative coupling for free alcohols using Deoxazole-quat, a Ni catalyst, and [Ir(dtbbpy)(ppy)2]PF6.
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Zhe Dong et al.
Nature, 598(7881), 451-456 (2021-09-01)
Metal-catalysed cross-couplings are a mainstay of organic synthesis and are widely used for the formation of C-C bonds, particularly in the production of unsaturated scaffolds1. However, alkyl cross-couplings using native sp3-hybridized functional groups such as alcohols remain relatively underdeveloped2. In particular
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service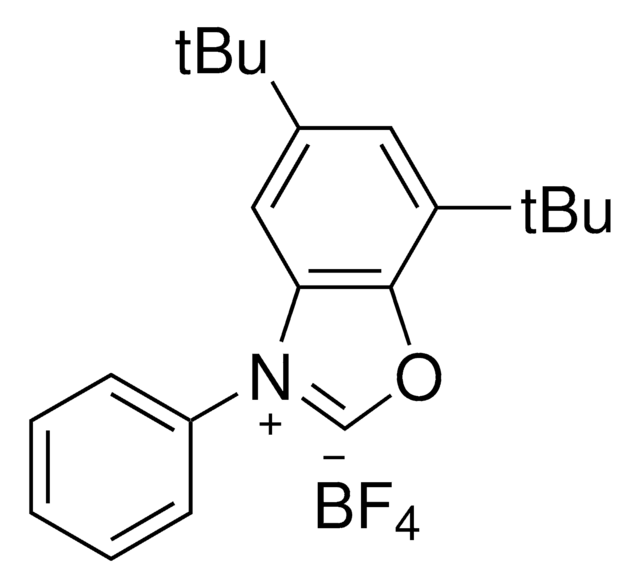
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)

![[Ir(dF(Me)ppy)2(dtbbpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/150/099/7c2dfa31-39f4-4cca-aee5-86d4a89fea78/640/7c2dfa31-39f4-4cca-aee5-86d4a89fea78.png)