806536
APN-Maleimide
Synonym(s):
3-(4-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)phenyl)propiolonitrile, MAPN
About This Item
Recommended Products
form
powder
Quality Level
storage temp.
2-8°C
SMILES string
N#CC#CC1=CC=C(N2C(C=CC2=O)=O)C=C1
InChI
1S/C13H6N2O2/c14-9-1-2-10-3-5-11(6-4-10)15-12(16)7-8-13(15)17/h3-8H
InChI key
CHKKXKRQICWZFF-UHFFFAOYSA-N
Application
Preparation Note
- Dissolve the protein in the appropriate buffer* with pH 6.5-9.0 (e.g. PBS) at 1-10 mg/mL concentration.
- Apply the appropriate amount of the stock solution of the reagent (1-5 molar eq. per free cysteine residue).
- Incubate at room temperature for 10 minutes.
- If necessary, purify the protein-APN conjugate using size exclusion chromatography or ultrafiltration.
- The conjugate can be readily coupled with thiol-containing substrates by incubating the components in aqueous buffer (pH 6.5-9.0) at ambient temperature for 2 hours.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service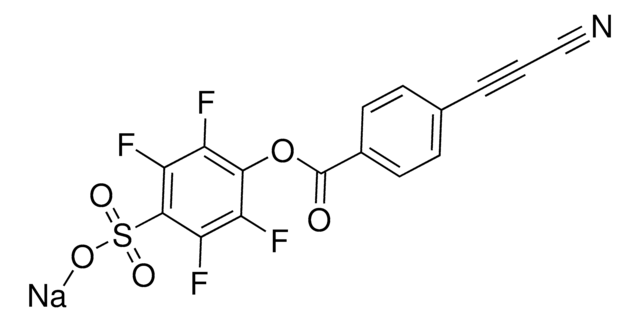


![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)