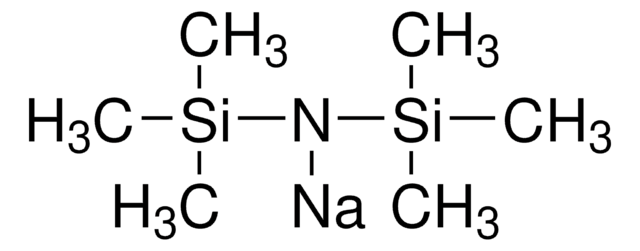80631
Sodium bis(trimethylsilyl)amide solution
40% in THF
Synonym(s):
HMN-Na Sol, NaHMDS solution, Hexamethyldisilazane sodium salt solution
About This Item
Recommended Products
form
liquid
concentration
40% in THF
SMILES string
C[Si](C)(C)N([Na])[Si](C)(C)C
InChI
1S/C6H18NSi2.Na/c1-8(2,3)7-9(4,5)6;/h1-6H3;/q-1;+1
InChI key
WRIKHQLVHPKCJU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Sodium bis(trimethylsilyl)amide, also known as NaHMDS, is a metal dialkylamide reagent that combines both high basicity and nucleophilicity. It is a general reagent used for the enolization of carbonyl compounds.
- It can be used to prepare phosphonium ylides in Wittig reaction.
- NaHMDS solution can also be used as a source of nitrogen in aminomethylation and in the synthesis of primary amines from the corresponding alkyl halides.
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
1.4 °F - closed cup
flash_point_c
-17 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service