792594
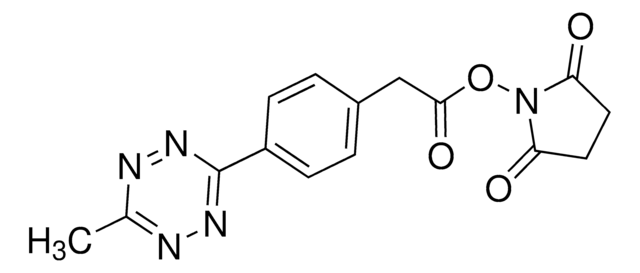
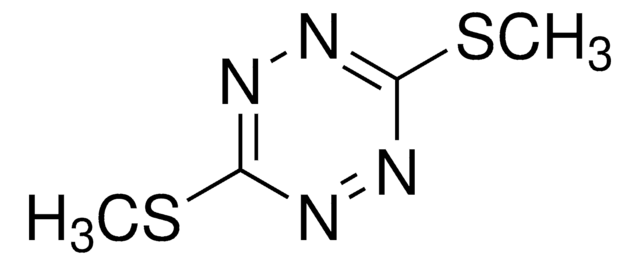
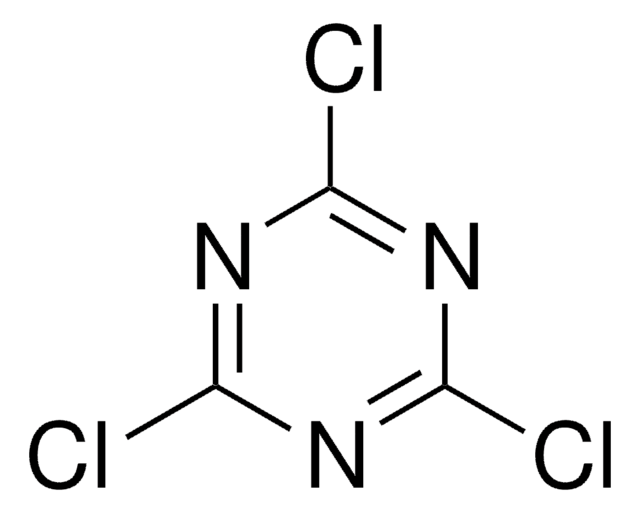
3,6-Dichloro-1,2,4,5-tetrazine
96%
Synonym(s):
Dichloro-s-tetrazine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C2Cl2N4
Molecular Weight:
150.95
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
form
solid
availability
available only in USA
mp
146-151 °C
storage temp.
2-8°C
SMILES string
ClC1=NN=C(Cl)N=N1
InChI
1S/C2Cl2N4/c3-1-5-7-2(4)8-6-1
InChI key
HXXFMIAFWKZHDY-UHFFFAOYSA-N
Application
Reagent has been reported in stapling of complex peptides as a photochemical trigger. Professor Amos Smith and coworkers have displayed this application in several recent reports.
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Self-react. C - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mohannad Abdo et al.
Organic letters, 14(13), 3518-3521 (2012-06-27)
The design, solid-phase synthesis, and photochemical validation of diverse peptide linchpins, containing the S,S-tetrazine phototrigger, have been achieved. Steady state irradiation or femtosecond laser pulses confirm their rapid photofragmentation. Attachment of peptides to the C- and N-termini will provide access
Joel R Courter et al.
The Journal of organic chemistry, 79(2), 759-768 (2013-12-24)
The design and synthesis of alanine-rich α-helical peptides constrained in a partially unfolded state by incorporation of the S,S-tetrazine phototrigger has been achieved, permitting, upon photochemical release, observation by 2D-IR spectroscopy of the subnanosecond conformational dynamics that govern the early
Stephen P Brown et al.
Journal of the American Chemical Society, 137(12), 4034-4037 (2015-03-21)
Protocols have been achieved that permit facile introduction of s-tetrazine into unprotected peptides and the protein, thioredoxin, between two cysteine sulfhydryl groups (i.e., staple), followed by photochemical release (i.e., unstaple) and regeneration of the peptide/protein upon removal of the cyano
Tetrazine phototriggers: probes for peptide dynamics.
Matthew J Tucker et al.
Angewandte Chemie (International ed. in English), 49(21), 3612-3616 (2010-04-15)
Matthew J Tucker et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(43), 17314-17319 (2013-10-10)
The relaxation of helical structures very close to equilibrium is observed via transient 2D IR spectroscopy. An initial distribution of synthetically distorted helices having an unnatural bridge linking the 10th and 12th residues of an alanine-rich α-helix is released to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1H-1,2,3-Triazolo[4,5-b]pyridine 98%](/deepweb/assets/sigmaaldrich/product/structures/344/744/1e7fa2cf-1258-48e0-909f-92509981f43d/640/1e7fa2cf-1258-48e0-909f-92509981f43d.png)