779385
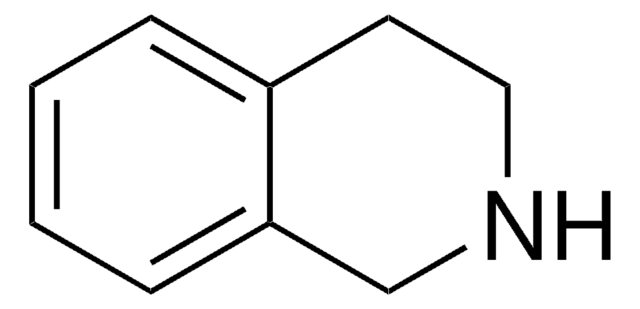
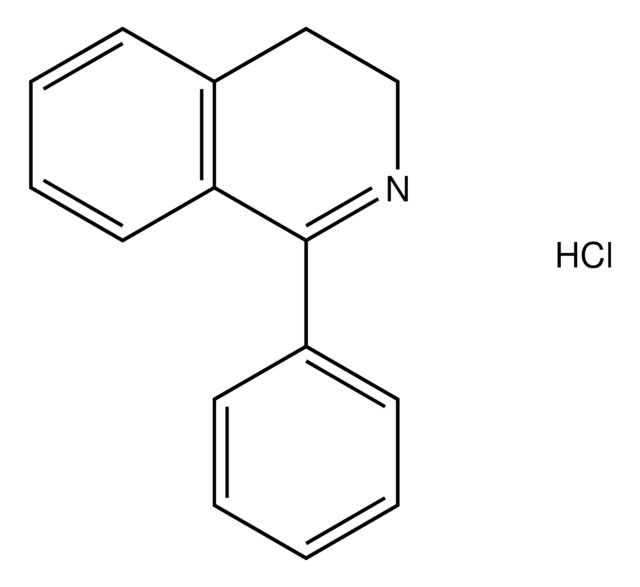
3,4-Dihydroisoquinoline
≥97.5% (GC)
Synonym(s):
3,4-Dihydroisoquinoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H9N
CAS Number:
Molecular Weight:
131.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥97.5% (GC)
97.5-102.5% (T)
form
solid
suitability
complies for identity (IR)
SMILES string
C1Cc2ccccc2C=N1
InChI
1S/C9H9N/c1-2-4-9-7-10-6-5-8(9)3-1/h1-4,7H,5-6H2
InChI key
NKSZCPBUWGZONP-UHFFFAOYSA-N
Application
3,4-Dihydroisoquinoline can be used as a reactant to synthesize:
- 5,6-Dihydro-8H-isoquino[1,2-b]quinazolin-8-one by decarboxylative cyclization reaction with isatoic anhydride using tetrabutylammonium iodide (TBAI).
- 1-naphtholyl tetrahydroisoquinoline by aza-Friedel-Crafts reaction with various naphthols.
- 3,4-dihydroisoquinoline pseudo bases, which are employed as starting materials for the preperation of 3-benzazepine derivatives.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Electrosynthesis of polycyclic quinazolinones and rutaecarpine from isatoic anhydrides and cyclic amines
Chen Xingyu, et al.
Royal Society of Chemistry Advances, 10(72), 44382-44386 (2020)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service