696641
3,3-Dimethyl-1-(trifluoromethyl)-1,2-benziodoxole
95%
Synonym(s):
1,3-Dihydro-3,3-dimethyl-1-(trifluoromethyl)-1,2-benziodoxole, Togni’s Reagent
About This Item
Recommended Products
Quality Level
assay
95%
form
powder
reaction suitability
reaction type: C-C Bond Formation
mp
75-79 °C
functional group
fluoro
iodo
storage temp.
2-8°C
SMILES string
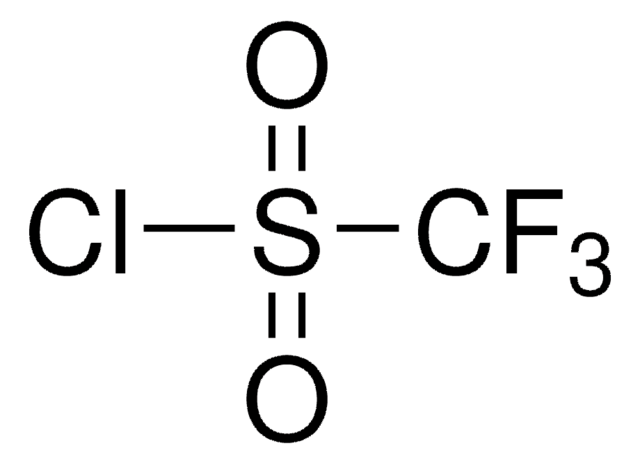
CC1(C)O[I](c2ccccc12)C(F)(F)F
InChI
1S/C10H10F3IO/c1-9(2)7-5-3-4-6-8(7)14(15-9)10(11,12)13/h3-6H,1-2H3
InChI key
HVAPLSNCVYXFDQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Trifluoromethylation of a variety of compounds including:
- Secondary and primary aryl- and alkylphospines
- Phenols
- Peptides containing cysteine residudes by SPPS and electrophilic S-trifluoromethylation
- Arenes and N-heterocycles
- Electrophilic N-trifluoromethylation of Arozoles
related product
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
4.1B - Flammable solid hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
The fluoroalkylation toolbox now includes Togni reagents, hypervalent iodine perfluoroalkylation reagents, fluoroalkyl bromides, silanes, carboxylates, and sulfonyl fluorides for late stage fluoroalkylation.
Related Content
Research in the Togni group focuses on the development of new ligands and reagents. These two general directions thus impact the ability to construct molecules in more efficient or unprecedented ways.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service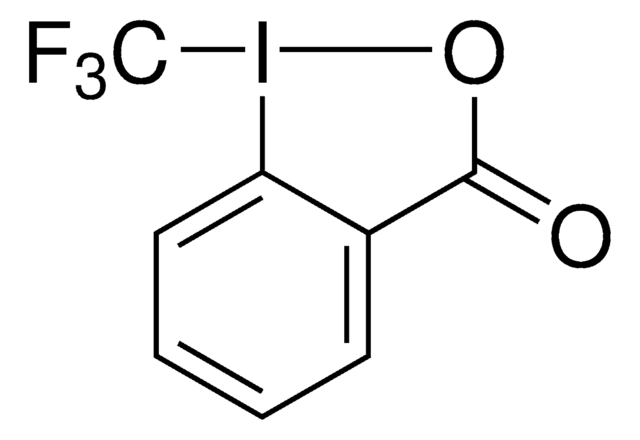



![1-[(Triisopropylsilyl)ethynyl]-1,2-benziodoxol-3(1H)-one ≥98.0% (AT)](/deepweb/assets/sigmaaldrich/product/structures/306/080/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d/640/5d7d692f-a7c1-44ce-a2f9-ee6cffe8f42d.png)