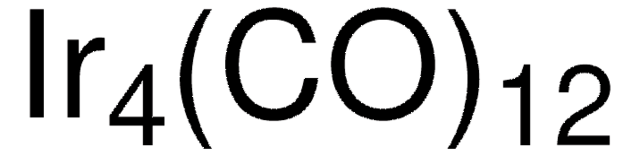683094
HS157
Umicore, 97%
Synonym(s):
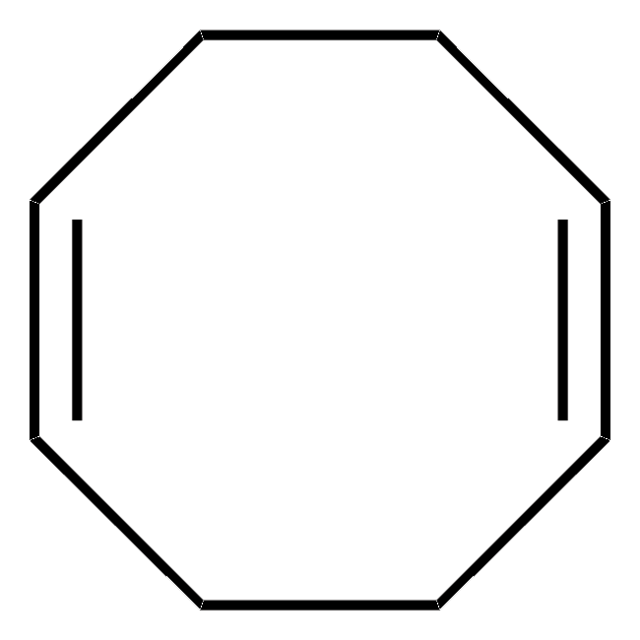
Bis(1,5-cyclooctadiene)diiridium(I) dichloride, [Ir(1,5-cod)Cl]2, 1,5-Cyclooctadiene-iridium(I) chloride dimer, Chloro(1,5-cyclooctadiene)iridium(I) dimer, Di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]diiridium, Iridium(I) chloride 1,5-cyclooctadiene complex dimer, [Ir(1,5-cod)Cl]2, [Ir(cod)Cl]2
About This Item
Recommended Products
Quality Level
assay
97%
form
powder
reaction suitability
core: iridium
reaction type: C-H Activation
reagent type: catalyst
mp
205 °C (dec.) (lit.)
SMILES string
Cl[Ir].Cl[Ir].C1CC=CCCC=C1.C2CC=CCCC=C2
InChI
1S/2C8H12.2ClH.2Ir/c2*1-2-4-6-8-7-5-3-1;;;;/h2*1-2,7-8H,3-6H2;2*1H;;/q;;;;2*+1/p-2/b2*2-1-,8-7-;;;;
InChI key
ZFOUDQNHNLDNLD-MIXQCLKLSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Metal precursor for asymmetric allylic substitutions.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
We are happy to offer a series of precatalysts for asymmetric catalysis and Pd based cross coupling catalysis. Our partnership with Umicore allows us to offer a portfolio of metal complexes with batch-to-batch consistency for a plethora of catalytic reactions.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 683094-10G | |
| 683094-1G | |
| 683094-500MG | 4061832755359 |
| 683094-2G | 4061832755335 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service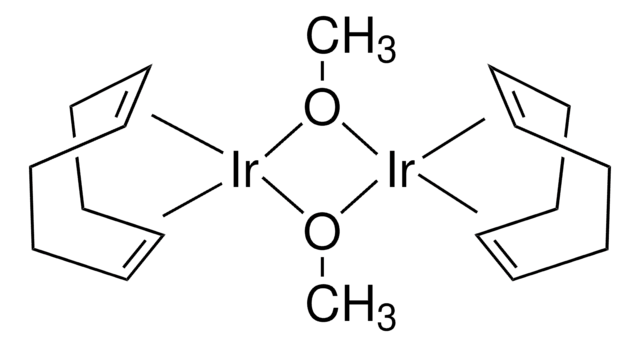



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)