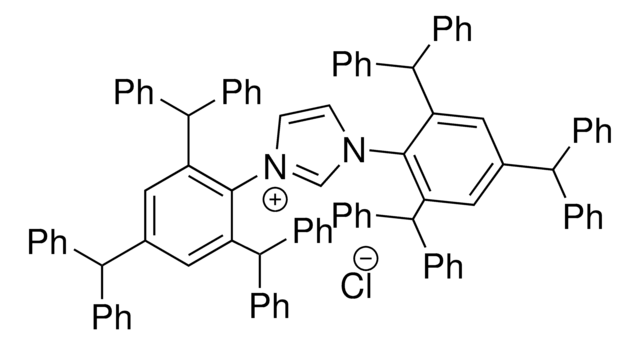
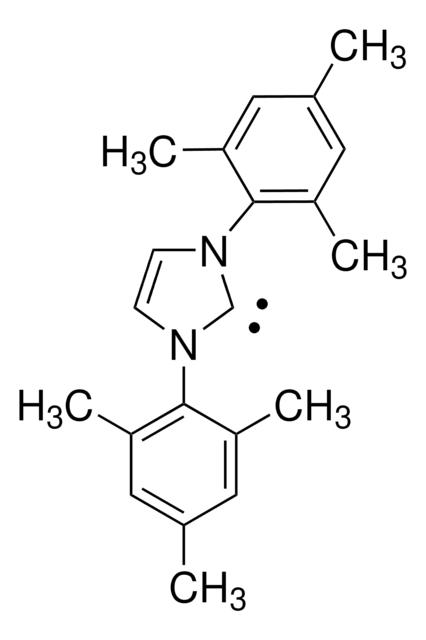
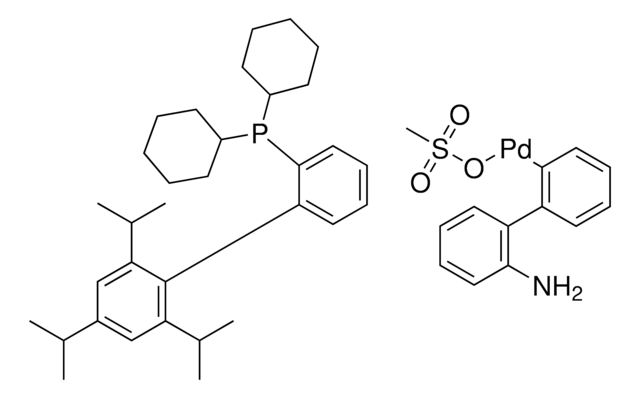
662232
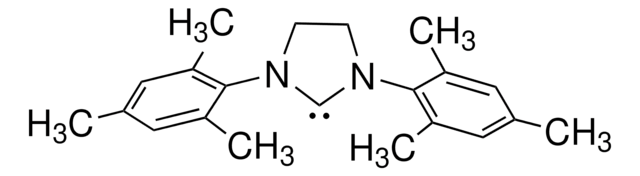
N-Heterocyclic Carbene Ligands Kit I
Synonym(s):
Heterocyclic Carbene Ligands
About This Item
Recommended Products
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
Quality Level
Kit Components Also Available Separately
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
A wide range of NHC ligands are commonly available which exhibit high activities.
Rapid progress in cross-coupling reactions of unactivated substrates catalyzed by metal complexes has transformed the chemical market-place through introduction of an extensive library of achiral and chiral phosphine ligands.
In collaboration with Umicore AG and Co.,1 we are pleased to offer a series of robust Pd(II) and Pd(0) complexes employed as the linchpin in C–C bond forming reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service