656364
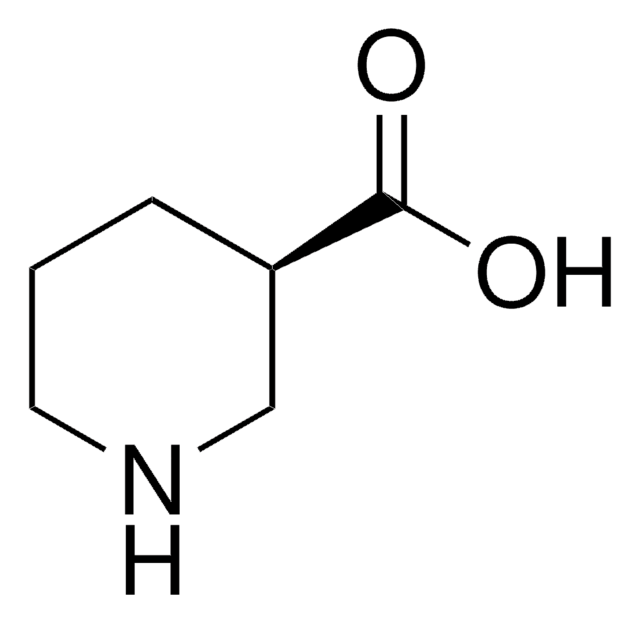
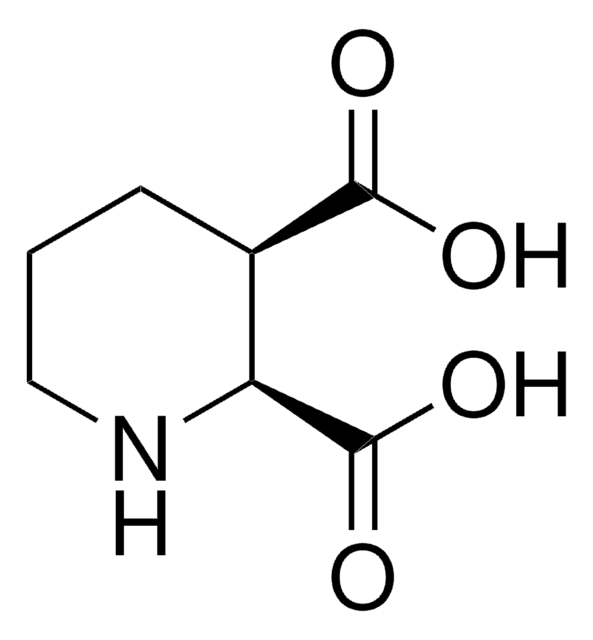
(S)-(+)-3-Piperidinecarboxylic acid
97%
Synonym(s):
(S)-(+)-Nipecotic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11NO2
CAS Number:
Molecular Weight:
129.16
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
solid
optical activity
[α]/D 3.0 to 6.5°, c = 1 in H2O
mp
254 °C (dec.)
functional group
carboxylic acid
SMILES string
OC(=O)[C@H]1CCCNC1
InChI
1S/C6H11NO2/c8-6(9)5-2-1-3-7-4-5/h5,7H,1-4H2,(H,8,9)/t5-/m0/s1
InChI key
XJLSEXAGTJCILF-YFKPBYRVSA-N
Gene Information
rat ... Slc6a1(79212)
Looking for similar products? Visit Product Comparison Guide
General description
(S)-(-)-3-Piperidinecarboxylic acid is an inhibitor of GABA (γ-aminobutyric acid) uptake.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Hong Gao et al.
Neuropharmacology, 58(8), 1220-1227 (2010-03-17)
Zolpidem is a widely prescribed sleep aid with relative selectivity for GABA(A) receptors containing alpha1-3 subunits. We examined the effects of zolpidem on the inhibitory currents mediated by GABA(A) receptors using whole-cell patch-clamp recordings from DMV neurons in transverse brainstem
Joanna R Dodd et al.
The Journal of biological chemistry, 282(21), 15528-15533 (2007-04-03)
The creatine transporter (CRT) is a member of a large family of sodium-dependent neurotransmitter and amino acid transporters. The CRT is closely related to the gamma-aminobutyric acid (GABA) transporter, GAT-1, yet GABA is not an effective substrate for the CRT.
Michael P DeNinno et al.
Bioorganic & medicinal chemistry letters, 21(10), 3095-3098 (2011-04-05)
The first highly potent and selective PDE8 inhibitors are disclosed. The initial tetrahydroisoquinoline hit was transformed into a nipecotic amide series in order to address a reactive metabolite issue. Reduction of lipophilicity to address metabolic liabilities uncovered an interesting diastereomer-dependent
Philip M Duy et al.
Experimental physiology, 95(7), 774-787 (2010-04-27)
Hyperthermic prolongation of the laryngeal chemoreflex (LCR) in decerebrate piglets is prevented or reversed by GABA(A) receptor antagonists and adenosine A(2A) (Ad-A(2A)) receptor antagonists administered in the nucleus of the solitary tract (NTS). Therefore, we tested the hypothesis that enhanced
Sabrina S Jedlicka et al.
International journal of neural systems, 19(3), 197-212 (2009-07-04)
In this work we quantified the in vitro calibration relationships between high frequency electrical stimulation and GABA and glutamate release in both mature retinoic acid differentiated P19 neurons and immortalized embryonic cortical cells engineered to express glutamic acid decarboxylase, GAD65.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service