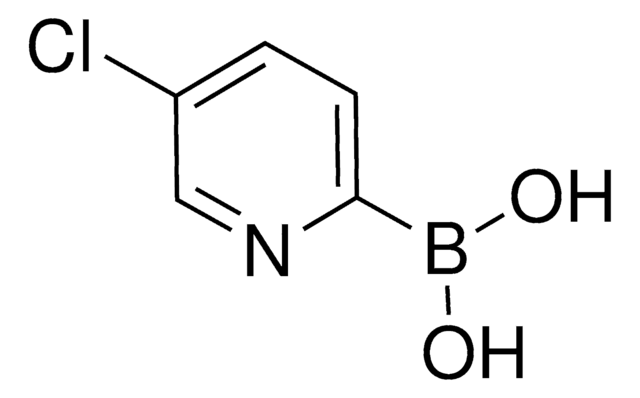
637386
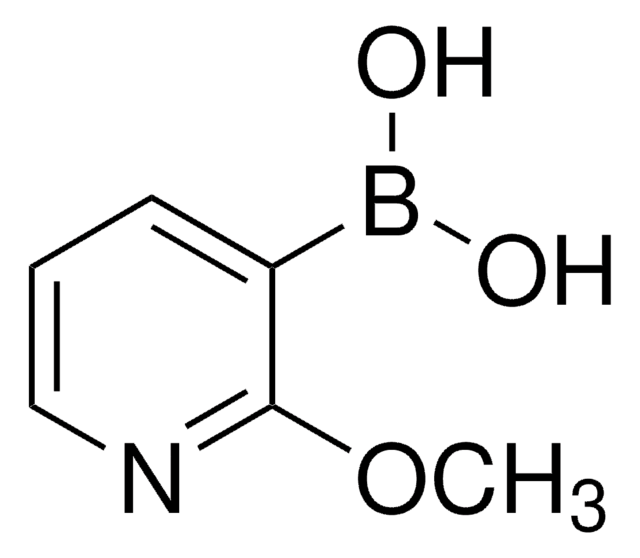
6-Chloro-3-pyridinylboronic acid
≥95.0%
Synonym(s):
2-Chloro-5-pyridineboronic acid
About This Item
Recommended Products
assay
≥95.0%
form
solid
mp
165 °C (lit.)
functional group
chloro
SMILES string
OB(O)c1ccc(Cl)nc1
InChI
1S/C5H5BClNO2/c7-5-2-1-4(3-8-5)6(9)10/h1-3,9-10H
InChI key
WPAPNCXMYWRTTL-UHFFFAOYSA-N
Application
- To prepare biologically significant 3-arylcoumarins by reacting with 3-chlorocoumarin through Suzuki reaction.
- As a substrate in the synthesis of 11-(pyridinylphenyl)steroid with progesterone agonist/antagonist profile.
- As a substrate in the preparation of α- secondary and tertiary pyridines by the reaction of pyridotriazoles with boronic acids.
- As a substrate in the palladium-catalyzed α-arylation of saturated cyclic amines and N-methyl amines.
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
This brochure contains a comprehensive selection of boronic acids, boronic acid esters, diboron esters, and transition-metal catalysts useful for the Suzuki–Miyaura coupling reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service