All Photos(1)
About This Item
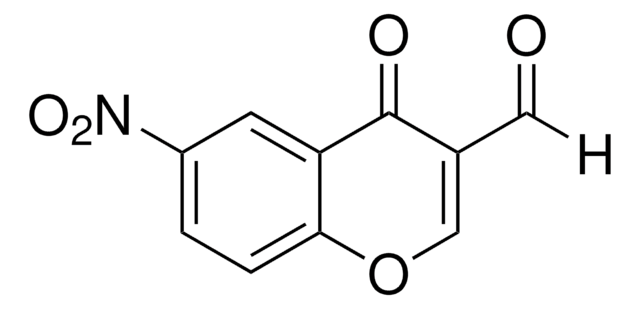
Empirical Formula (Hill Notation):
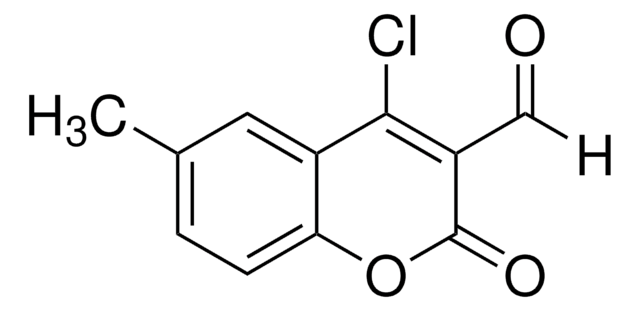
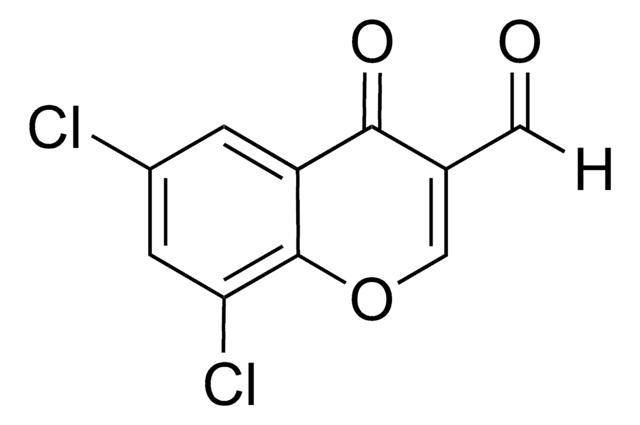
C10H5ClO3
CAS Number:
Molecular Weight:
208.60
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
126-130 °C (lit.)
functional group
aldehyde
chloro
ester
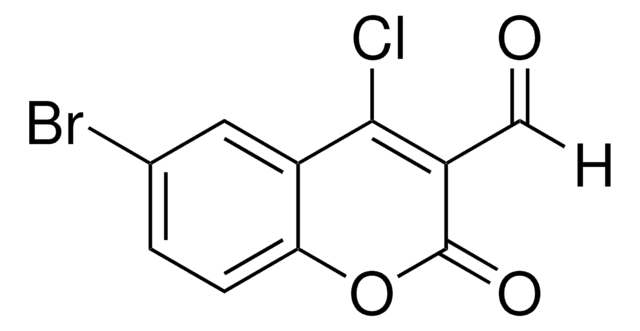
SMILES string
[H]C(=O)C1=C(Cl)c2ccccc2OC1=O
InChI
1S/C10H5ClO3/c11-9-6-3-1-2-4-8(6)14-10(13)7(9)5-12/h1-5H
InChI key
CLLLQUGVEQADNN-UHFFFAOYSA-N
General description
4-Chloro-3-formylcoumarin, also known as 4-chloro-2-oxo-2H-chromene-3-carbaldehyde, is a coumarin derivative.
Application
4-Chloro-3-formylcoumarin may be used as a reactant in the preparation of:
- 2-aryl[1]benzopyrano[4,3-c]pyrazol-4(2H)-ones
- biaryl lactones (benzo[c]chromen-6-ones)
- N-monosubstituted 4-amino-3-formylcoumarins
- chromeno[4,3-b]quinolin-6-one
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 6H-benzo [c] chromen-6-ones by cyclocondensation of 1,3-dicarbonyl compounds with 4-chloro-3-formylcoumarin.
Iaroshenko, VO, et al.
Tetrahedron Letters, 52(45), 5910-5912 (2011)
An efficient ultrasound promoted catalyst-free protocol for the synthesis of chromeno [4,3-b] quinolin-6-ones.
Prasad JV, et al.
Chemical Science, 123(5), Prasad JV-Prasad JV (2011)
Reactions of 4-Chloro-3-formylcoumarin with Arylhydrazines.
Strakova I, et al.
Chemistry of Heterocyclic Compounds, 39(12), 1608-1616 (2003)
Reactions of 4-chloro-3-formyl-coumarin with primary amines.
Strakova I, et al.
Chemistry of Heterocyclic Compounds, 42(5), 574-582 (2006)
One-Pot Synthesis of Biaryl Lactones by Sonogashira Cross-Coupling Reactions of 4-Chloro-3-formylcoumarin and Subsequent Domino [5+1] Cyclization/Deacetylation Reactions with 1, 3-Dicarbonyl Compounds.
Iaroshenko VO, et al.
Advanced Synthesis & Catalysis, 354(5), 803-806 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service