531480
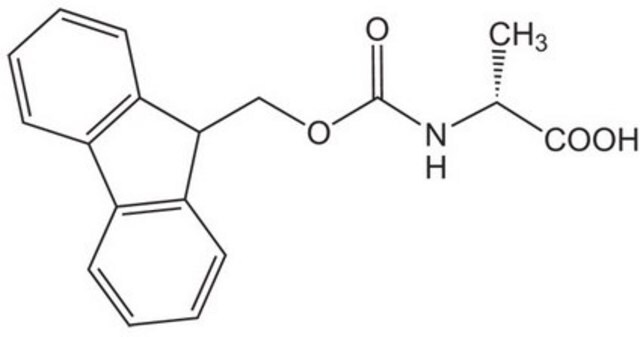
Fmoc-Ala-OH
95%, for peptide synthesis
Synonym(s):
N-(9-Fluorenylmethoxycarbonyl)-L-alanine, Fmoc-L-alanine
About This Item
Recommended Products
Product Name
Fmoc-Ala-OH, 95%
Quality Level
assay
95%
optical activity
[α]20/D −18°, c = 1 in DMF
reaction suitability
reaction type: C-H Activation
reaction type: Fmoc solid-phase peptide synthesis
reagent type: ligand
reaction type: Peptide Synthesis
mp
147-153 °C (lit.)
application(s)
peptide synthesis
functional group
Fmoc
amine
carboxylic acid
storage temp.
2-8°C
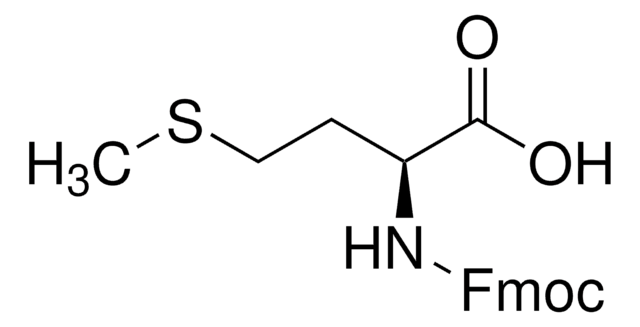
SMILES string
C[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI
1S/C18H17NO4/c1-11(17(20)21)19-18(22)23-10-16-14-8-4-2-6-12(14)13-7-3-5-9-15(13)16/h2-9,11,16H,10H2,1H3,(H,19,22)(H,20,21)/t11-/m0/s1
InChI key
QWXZOFZKSQXPDC-NSHDSACASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a building block in the preparation of triazolopeptides , and azapeptides
- in the synthesis of bis-cationic porphyrin peptides using the standard Fmoc solid-phase synthesis
- to transform Mannich-adducts into α-halogenated amides without undergoing aziridination
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
With a growing peptide drug market the fast, reliable and uncomplicated synthesis of peptides is of paramount importance.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 531480-100G | 4061832558714 |
| 531480-1KG | 4061832642710 |
| 531480-25G | 4061832558721 |
| 531480-5G | 4061832558738 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service