All Photos(1)
About This Item
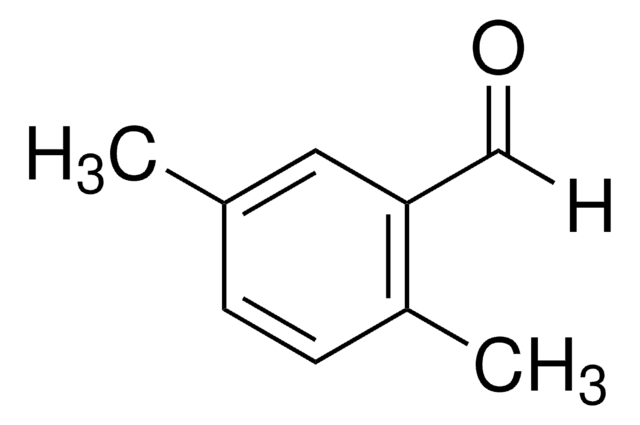
Linear Formula:
(CH3)2C6H3CHO
CAS Number:
Molecular Weight:
134.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
refractive index
n20/D 1.551 (lit.)
bp
226 °C (lit.)
density
1.012 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
Cc1ccc(C=O)cc1C
InChI
1S/C9H10O/c1-7-3-4-9(6-10)5-8(7)2/h3-6H,1-2H3
InChI key
POQJHLBMLVTHAU-UHFFFAOYSA-N
General description
3,4-Dimethylbenzaldehyde (3,4-DMB) is a benzaldehyde derivative. It is the OH radical initiated oxidative degradation product of trimethylbenzene. The rate coefficient of the gas-phase reaction between 3,4-DMB and OH radical is 24.6±4.0×10-12cm3molecule-1s-1. The vibrational analysis of 3,4-DMB based on FT-IR spectra, FT-Raman spectra, ab initio and density functional theory (DFT) calculations have been reported. It is formed as an intermediate during the transformation of furfural into gasoline-range fuels using ZSM(Zeolite Socony Mobil)-5-based catalysts.
Application
3,4-Dimethylbenzaldehyde may be used in the synthesis of 3,4-dimethylmethcathinone (DMMC) and 3,4-dimethyl-dibenzylidene sorbitol.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vibrational spectroscopy investigation using ab initio and density functional theory analysis on the structure of 3, 4-dimethylbenzaldehyde.
Sundaraganesan N, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 68(3), 680-687 (2007)
Catalytic fast pyrolysis of furfural over H-ZSM-5 and Zn/H-ZSM-5 catalysts.
Fanchiang WL and Lin YC.
Applied Catalysis A: General, 419, 102-110 (2012)
Synthesis of 3,4-Dimethyl-dibenzylidene Sorbitol at Room Temperature.
Yin ZY, et al.
Huaxue Shiji, 47(3), 174-174 (2006)
Urinary excretion and metabolism of the newly encountered designer drug 3,4-dimethylmethcathinone in humans.
Shima N, et al.
Forensic Toxicology, 31(1), 101-112 (2013)
Rate coefficients for the gas-phase reaction of hydroxyl radicals with the dimethylbenzaldehydes.
Clifford GM and Wenger JC.
International Journal of Chemical Kinetics, 38(9), 563-569 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service