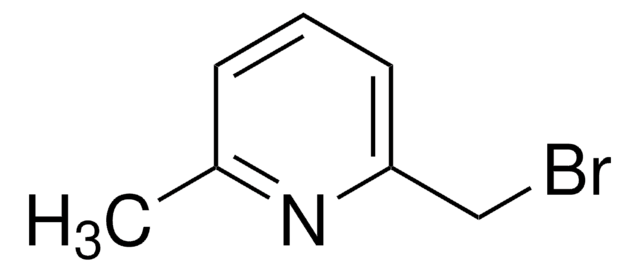
491748
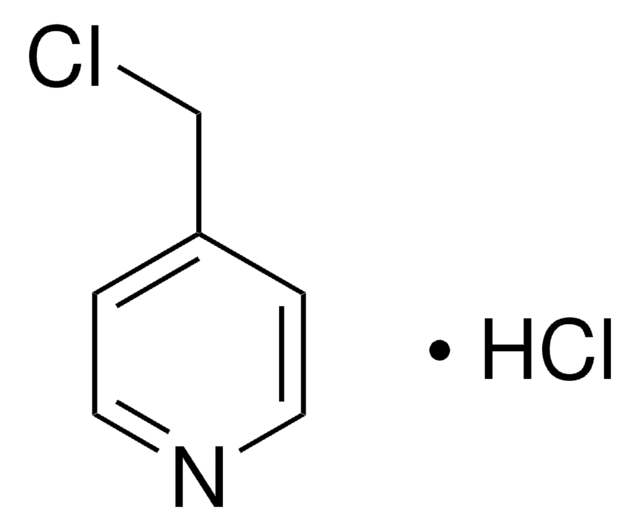
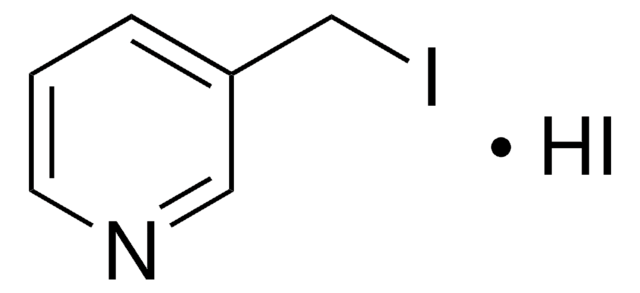
4-(Bromomethyl)pyridine hydrobromide
97%
Synonym(s):
(4-Pyridyl)methyl bromide hydrobromide, 4-(Bromomethyl)pyridine monohydrobromide, 4-Picolyl bromide hydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H6BrN · HBr
CAS Number:
Molecular Weight:
252.93
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
mp
189-192 °C (lit.)
functional group
bromo
SMILES string
Br[H].BrCc1ccncc1
InChI
1S/C6H6BrN.BrH/c7-5-6-1-3-8-4-2-6;/h1-4H,5H2;1H
InChI key
VAJUUDUWDNCECT-UHFFFAOYSA-N
General description
4-(Bromomethyl)pyridine hydrobromide is a substituted pyridine. It reacts with 1,2-ethanediamine and 1,3-propanediamine to form the corresponding diamines.
Application
4-(Bromomethyl)pyridine hydrobromide may be used in the preparation of:
- 3-(4-pyridylmethyl)-2′,3′-di-O-oleyl-5′-O-(4,4′-dimethoxytriphenylmethyl)uridine
- 3-(4-pyridylmethyl)-3′-O-oleyl-5′-O-(4,4-dimethoxytriphenylmethyl)-thymidine
- 1,4-bis(N-hexyl-4-pyridinium)butadiene diperchlorate
- 2-morpholin-4-yl-7-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
- 8-methyl-2-morpholin-4-yl-7-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
- 2-morpholin-4-yl-8-(pyridin-4-ylmethoxy)-4H-1,3-benzoxazin-4-one
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Luca Simeone et al.
Molecular bioSystems, 7(11), 3075-3086 (2011-09-08)
Novel thymidine- or uridine-based nucleolipids, containing one hydrophilic oligo(ethylene glycol) chain and one or two oleic acid residues (called ToThy, HoThy and DoHu), have been synthesized with the aim to develop bio-compatible nanocarriers for drug delivery and/or produce pro-drugs. Microstructural
Photoinduced electron transfer in supramolecular complexes of a p-extended viologen with porphyrin monomer and dimer.
Fukuzumi S, et al.
Royal Society of Chemistry Advances, 2(9), 3741-3747 (2012)
Cristiane F da Costa et al.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 63(1), 40-42 (2008-02-12)
We report in this work the preparation and the in vitro antileishmanial activity of a series of long chains N-monoalkylated diamines and two pyridinediamine derivatives. Several compounds, tested for their in vitro antiproliferative activity against Leishmania amazonensis and Leishmania chagasi
Saleh Ihmaid et al.
European journal of medicinal chemistry, 45(11), 4934-4946 (2010-08-31)
A number of new 2-amino-[5, 6, 7 and 8]-O-substituted 1,3-benzoxazines, and 2-amino 8-methyl-7-O-substituted-1,3-benzoxazines were synthesized. Thirty one new compounds were tested for their effect on collagen induced platelet aggregation and it was found that the most active compounds were 8-methyl-2-morpholin-4-yl-7-(pyridin-3-ylmethoxy)-4H-1,3-benzoxazin-4-one
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service