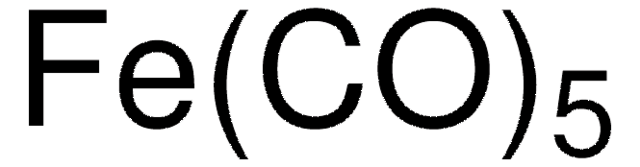481718
Iron(0) pentacarbonyl
>99.99% trace metals basis
Synonym(s):
Iron(0) carbonyl, Pentacarbonyliron(0)
About This Item
Recommended Products
vapor density
6.74 (vs air)
vapor pressure
35 mmHg ( 25 °C)
40 mmHg ( 30.3 °C)
assay
>99.99% trace metals basis
form
liquid
autoignition temp.
122 °F
reaction suitability
core: iron
reagent type: catalyst
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
refractive index
n20/D 1.5196 (lit.)
bp
103 °C (lit.)
mp
−20 °C (lit.)
density
1.49 g/mL at 25 °C (lit.)
greener alternative category
, Aligned
storage temp.
2-8°C
SMILES string
[Fe].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+].[C-]#[O+]
InChI
1S/5CO.Fe/c5*1-2;
InChI key
FYOFOKCECDGJBF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To fabricate Fe-based anode materials for high-performance aqueous secondary Ni−Fe batteries. It helps to achieve good safety, high power density, long term-life cycle, and high energy density.
- As a precursor to synthesize highly stable, colloidal magnetic iron nanoparticles with narrow size distribution. These nanoparticles are potential candidates for MRI contrasting agents and magnetically separable catalysts.
- To synthesize zero valent sustainable iron nanocatalysts for reduction reactions.
- Addition reactions
- Decomposition via photolysis
- Preparation of magnetic Fe onion-like fullerene micrometer-sized particles of narrow size distribution via thermal decomposition
- Preparation of a heptanuclear iron carbonyl cluster used in catalytic hydrosilane reduction of carboxamides
- Precursor for preparation of Fe nanoparticles for photocatalytic hydrogen evolution under highly basic conditions
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
flash_point_f
5.0 °F - closed cup
flash_point_c
-15 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Solvothermal synthesis is a method for preparing a variety of materials such as metals, semiconductors, ceramics, and polymers.
Magnetic nanoparticles have attracted tremendous attention due to their novel properties and their potential applications in magnetic recording, magnetic energy storage and biomedicine.
Spin-based electronic (spintronic) devices offer significant improvement to the limits of conventional charge-based memory and logic devices which suffer from high power usage, leakage current, performance saturation, and device complexity.
The properties of many devices are limited by the intrinsic properties of the materials that compose them.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service