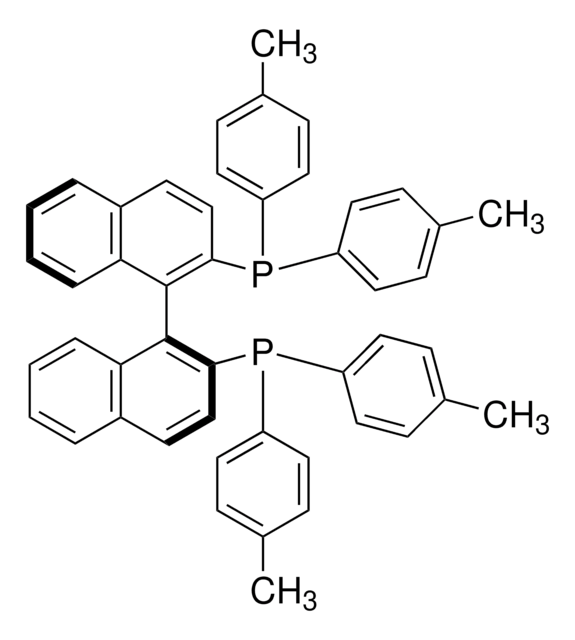
481084
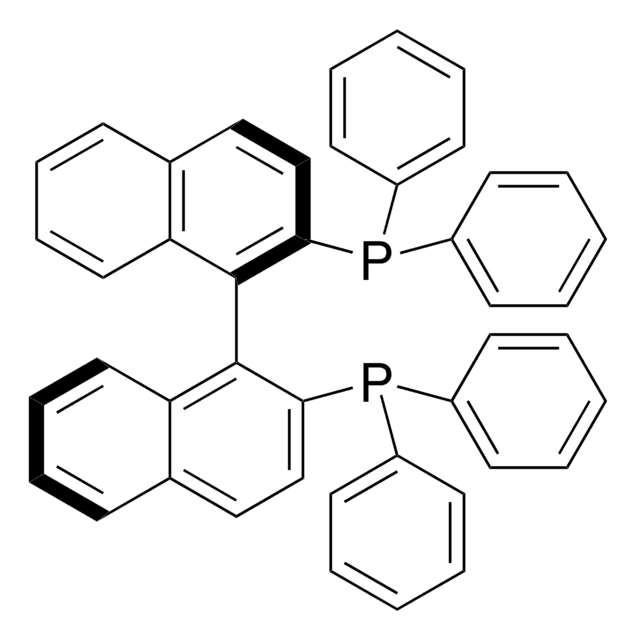
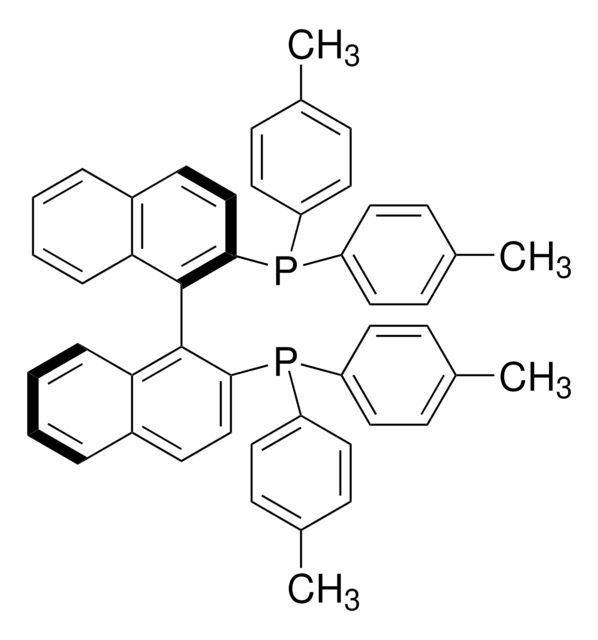
rac-BINAP
97%
Synonym(s):
(±)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene, 2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene, (±)-BINAP, [1,1′-Binaphthalene]-2,2′-diylbis[diphenylphosphine]
About This Item
Recommended Products
Quality Level
assay
97%
reaction suitability
reaction type: Cross Couplings
reagent type: ligand
reaction type: Acylations
reagent type: ligand
reaction type: Arylations
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: C-C Bond Formation
reagent type: ligand
reaction type: Decarboxylations
reagent type: ligand
reaction type: Stille Coupling
mp
283-286 °C (lit.)
functional group
phosphine
SMILES string
P(c8ccccc8)(c7ccccc7)c1c(c6c(cc1)cccc6)c2c3c(ccc2P(c5ccccc5)c4ccccc4)cccc3
InChI
1S/C44H32P2/c1-5-19-35(20-6-1)45(36-21-7-2-8-22-36)41-31-29-33-17-13-15-27-39(33)43(41)44-40-28-16-14-18-34(40)30-32-42(44)46(37-23-9-3-10-24-37)38-25-11-4-12-26-38/h1-32H
InChI key
MUALRAIOVNYAIW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 481084-100G | 4061833553305 |
| 481084-25G | 4061832388519 |
| 481084-500G | 4061833502709 |
| 481084-5G | 4061832388526 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service