All Photos(3)
About This Item
Linear Formula:
(H2C=CHCH2CH2CO)2O
CAS Number:
Molecular Weight:
182.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
refractive index
n20/D 1.447 (lit.)
bp
78-81 °C/0.4 mmHg (lit.)
density
0.997 g/mL at 25 °C (lit.)
functional group
allyl
anhydride
ester
SMILES string
C=CCCC(=O)OC(=O)CCC=C
InChI
1S/C10H14O3/c1-3-5-7-9(11)13-10(12)8-6-4-2/h3-4H,1-2,5-8H2
InChI key
NEDHQDYBHYNBIF-UHFFFAOYSA-N
Related Categories
General description
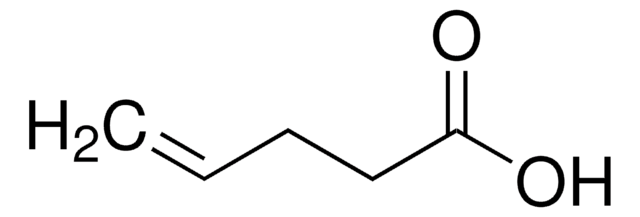
4-Pentenoic anhydride is a carboxylic anhydride.
Application
4-Pentenoic anhydride may be used:
- In the preparation of glucose functionalized (co)polymers.
- As a monomer in the preparation of cross-linked polyanhydrides.
- For the preparation of polymers with pendant vinyl or acetylene.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
230.0 °F - closed cup
flash_point_c
110 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-(Trimethylsilyl) ethyl Glycosides. Transformation into the Corresponding 1-O-Acyl Sugars.
Ellervik U and Magnusson G.
Acta Chemica Scandinavica, 47, 826-826 (1993)
Studies of the mechanism of the hypoglycemic action of 4-pentenoic acid.
Corredor C, et al.
Proceedings of the National Academy of Sciences of the USA, 58(6), 2299-2299 (1967)
Gaojian Chen et al.
Chemical communications (Cambridge, England), (10)(10), 1198-1200 (2009-02-26)
Homopolymer and block copolymer bearing carbohydrate side chain functionality were obtained by grafting glucothiose onto alkene functional scaffolds via a thiol-ene click reaction and the resulting copolymer was used to form thermo-responsive micelles as a potential drug carrier.
Katie L Poetz et al.
Biomacromolecules, 15(7), 2573-2582 (2014-05-23)
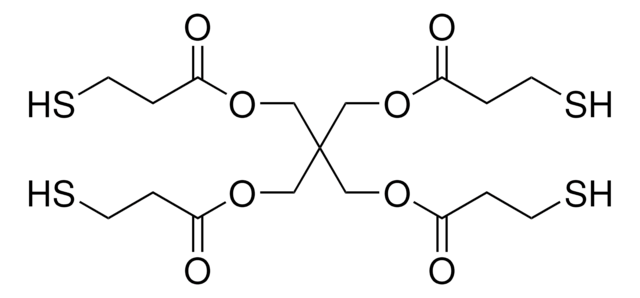
Several critical aspects of cross-linked polyanhydrides made using thiol-ene polymerization are reported, in particular the erosion, release, and solution properties, along with their cytotoxicity toward fibroblast cells. The monomers used to synthesize these polyanhydrides were 4-pentenoic anhydride and pentaerythritol tetrakis(3-mercaptopropionate).
Vien T Huynh et al.
Biomacromolecules, 12(5), 1738-1751 (2011-04-12)
Statistical and block copolymers based on poly(2-hydroxyethyl methacrylate) (PHEMA) and poly[oligo(ethylene glycol) methylether methacrylate] (POEGMEMA) were modified with 4-pentenoic anhydride or 4-oxo-4-(prop-2-ynyloxy)butanoic anhydride to generate polymers with pendant vinyl or acetylene, respectively. Subsequent thiol-ene or thiol-yne reaction with thioglycolic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service