All Photos(1)
About This Item
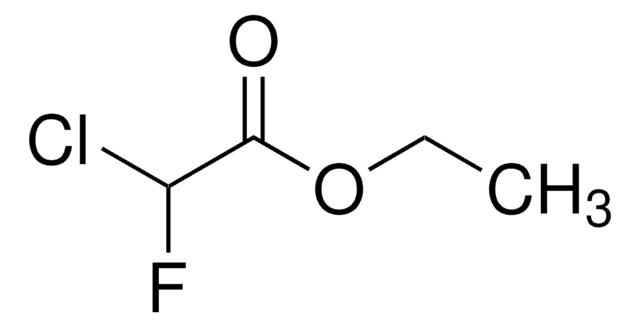
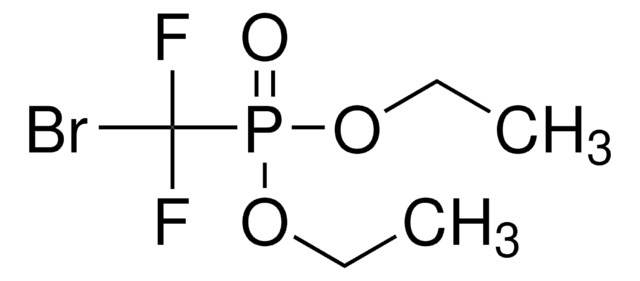
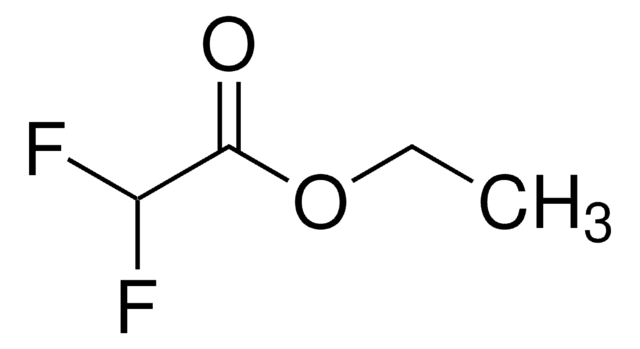
Linear Formula:
ClCF2CO2C2H5
CAS Number:
Molecular Weight:
158.53
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
refractive index
n20/D 1.358 (lit.)
bp
96-97.5 °C (lit.)
density
1.252 g/mL at 25 °C (lit.)
functional group
chloro
ester
fluoro
SMILES string
CCOC(=O)C(F)(F)Cl
InChI
1S/C4H5ClF2O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChI key
GVCAWQUJCHZRCB-UHFFFAOYSA-N
General description
Ethyl chlorodifluoroacetate (ECDFA) undergoes Reformatskii reaction with various aldehydes in DMF. It reacts with phenylacetylene to afford ethyl α,α−difluoro-4-phenyl-3-butenoates.
Application
Ethyl chlorodifluoroacetate (ECDFA) may be used as a starting reagent in the synthesis of 3-gem-difluoro-2-ethoxy allylic alcohols.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
64.4 °F - closed cup
flash_point_c
18 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fluorine-containing organozinc reagents. IV.: Reformatskii-type reactions of chlorodifluoroacet1c acid derivatives.
Lang RW and Schaub B.
Tetrahedron Letters, 29(24), 2943-2946 (1988)
Synthesis of 3-gem-difluoro-2-ethoxy allylic alcohols from ethyl chlorodifluoroacetate.
Begue J-P, et al.
Tetrahedron Letters, 35(3), 6097-6100 (1994)
A theoretical investigation on the kinetics and reactivity of the gas-phase reactions of ethyl chlorodifluoroacetate with OH radical and Cl atom at 298 K.
Mishra BK, et al.
Structural Chemistry, 25(2), 463-470 (2014)
Wadih Ghattas et al.
The Journal of organic chemistry, 71(22), 8618-8621 (2006-10-27)
An efficient preparation of pure ethyl Z- and E-alpha,alpha-difluoro-4-phenyl-3-butenoate 1a and 1b together with the corresponding acids 2a and 2b is described. The procedures involve stereocontrolled additions of *CF2CO2Et to phenylacetylene or beta-bromostyrene. Compound 1a is easily obtained by addition
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service