443271
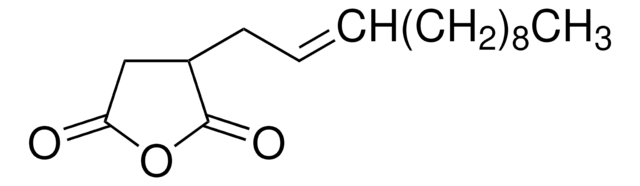
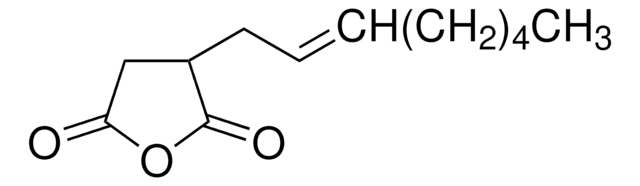
Dodecenylsuccinic anhydride, mixture of isomers
technical grade, 90%
Synonym(s):
DDSA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H26O3
CAS Number:
Molecular Weight:
266.38
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
technical grade
Quality Level
assay
90%
form
liquid
refractive index
n20/D 1.479 (lit.)
bp
150 °C/3 mmHg (lit.)
density
1.005 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCCC\C=C\C1CC(=O)OC1=O
InChI
1S/C16H26O3/c1-2-3-4-5-6-7-8-9-10-11-12-14-13-15(17)19-16(14)18/h11-12,14H,2-10,13H2,1H3/b12-11+
InChI key
WVRNUXJQQFPNMN-VAWYXSNFSA-N
General description
Dodecenylsuccinicanhydride (DDSA) is a mixture of isomers that belongs to the class of long-chained aliphatic anhydrides. It is composed of a dodecenyl (C12) chain attached to a succinic acid backbone and can undergo esterification, amidation, and ring-openingreactions. It is widely used as a cross-linking agent to synthesize hydrogels for drug delivery, coating for medical devices, and as a hardener for curing epoxy resins.
Application
Dodecenylsuccinic anhydride, mixture of isomers can be used:
- As a precursor to synthesize modified chitosan hydrogels useful for the sustained local delivery of drugs. This modification enhances the hydrophobicity of chitosan, allowing it to better encapsulate and retain hydrophobic drugs.
- To prepare high-performance Arabinoglucuronoxylan (AGX)-based biosurfactants using rapid homogeneous esterification reactions. These surfactants can be used for oily sludge remediation and oil recovery.
- As a hardener for curing thermosetting epoxy resins used in various fields such as paints, corrosion protective coatings, adhesives, and electrical circuits.
- As a modifying agent to prepare collagen hydrogels to fabricate skin wound dressings.
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 4 - Eye Irrit. 2 - Skin Sens. 1
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Glycine surfactants derived from dodecenyl succinic anhydride
Dix L and Moon A
Journal of Surfactants and Detergents, 15(3), 351-357 (2012)
Epoxy resins
Nixon R, et al.
Kanerva's Occupational Dermatology, 559-581 (2012)
Bancroft's Theory and Practice of Histological Techniques (2018)
A A Gorkun et al.
Biomedical materials (Bristol, England), 13(4), 044108-044108 (2018-05-04)
One of the essential goals in regenerative medicine is microvascularization which enables an effective blood supply within de novo constructed tissues and organs. In our study, we used two common multipotent mesenchymal stromal cell (MMSC) sources (subcutaneous adipose tissue and
Magnetic microreactors for efficient and reliable magnetic nanoparticle surface functionalization
Digigow RG, et al.
Lab on a chip, 14(13), 2276-2286 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service