425834
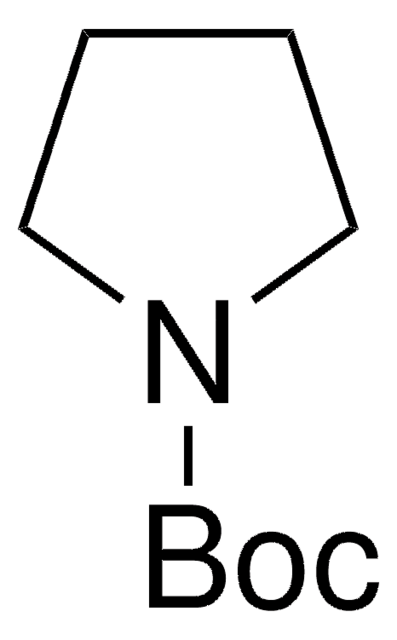
N-Boc-pyrrole
98%
Synonym(s):
tert-Butyl 1-pyrrolecarboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H13NO2
CAS Number:
Molecular Weight:
167.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.4685 (lit.)
bp
91-92 °C/20 mmHg (lit.)
density
1 g/mL at 25 °C (lit.)
SMILES string
CC(C)(C)OC(=O)n1cccc1
InChI
1S/C9H13NO2/c1-9(2,3)12-8(11)10-6-4-5-7-10/h4-7H,1-3H3
InChI key
IZPYBIJFRFWRPR-UHFFFAOYSA-N
Related Categories
General description
N-Boc-pyrrole is an N-protected pyrrole. It undergoes Diels–Alder reaction with enantiomerically pure allene-1,3-dicarboxylates to form endo-adducts with retention in configurations at two newly generated stereogenic centers. It also undergoes cyclopropanation with methyl phenyldiazoacetate to form both monocyclopropane and dicyclopropane. Its Ir-catalyzed C-H borylation followed by cross coupling with 3-chlorothiophene to form biheterocycle has been reported.
Application
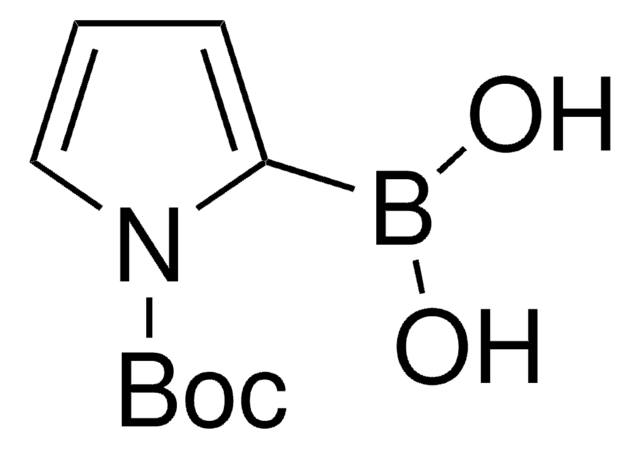
N-Boc-pyrrole was used in the synthesis of 1-(tert-butoxycarbonyl)-1H-pyrrol-2-ylboronic acid by treating with n-BuLi and subsequent reaction with trimethyl borate.
It may be used as starting material in the synthesis of the following:
It may be used as starting material in the synthesis of the following:
- tropane drivatives
- N-boc-2-(4-methoxyphenyl)pyrrole
- N-boc-pyrrol-2-ylboronic acid
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
167.0 °F - closed cup
flash_point_c
75 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic approaches to enantiomerically pure 8-azabicyclo [3.2. 1] octane derivatives.
Pollini GP, et al.
Chemical Reviews, 106(6), 2434-2454 (2006)
Huw M L Davies et al.
Chemical Society reviews, 38(11), 3061-3071 (2009-10-23)
The metal catalyzed reactions of diazo compounds have been broadly used in organic synthesis. The resulting metal-carbenoid intermediates are capable of undergoing a range of unconventional reactions, and due to their high energy, they are ideal for initiating cascade sequences
Recent progress in the synthesis of five-membered heterocycle boronic acids and esters.
Primas N, et al.
Tetrahedron, 66(41), 8121-8136 (2010)
Nikola Basarić et al.
Organic & biomolecular chemistry, 3(15), 2755-2761 (2005-07-21)
Two fluorescent off-on Ca2+ indicators based on APTRA (o-aminophenol-N,N,O-triacetic acid) as low-affinity ligand for Ca2+ and BODIPY(4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) as a fluorophore were synthesized. The new BODIPY-APTRA compounds absorb in the visible spectrum, with absorption maxima from 505 nm to 570 nm
CuO/SiO2 as a simple, effective and recoverable catalyst for alkylation of indole derivatives with diazo compounds.
Fraile JM, et al.
Organic & Biomolecular Chemistry, 11(26), 4327-4332 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service