All Photos(2)
About This Item
Linear Formula:
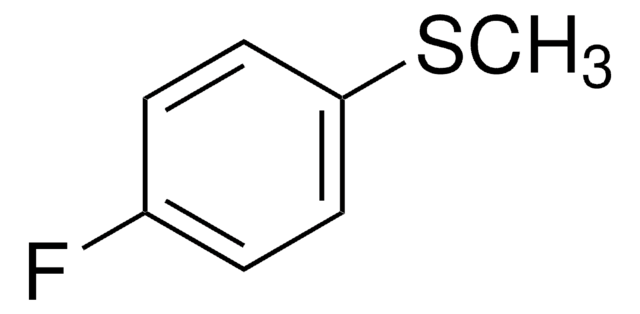
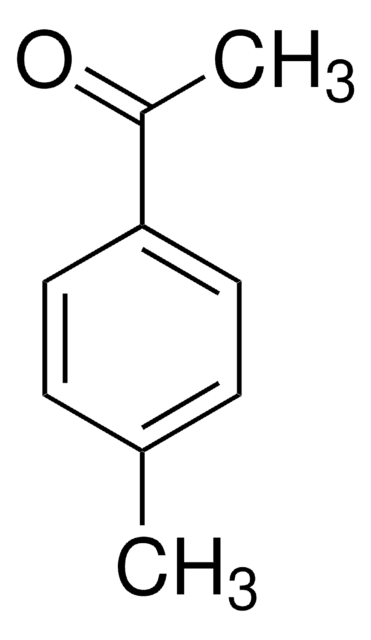
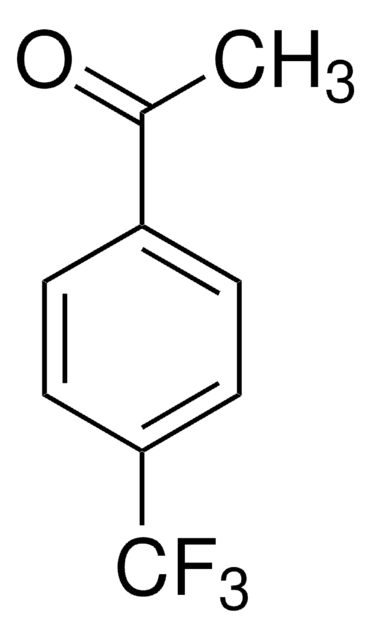
CH3SC6H4COCH3
CAS Number:
Molecular Weight:
166.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
mp
80-82 °C (lit.)
SMILES string
CSc1ccc(cc1)C(C)=O
InChI
1S/C9H10OS/c1-7(10)8-3-5-9(11-2)6-4-8/h3-6H,1-2H3
InChI key
JECUZQLBQKNEMW-UHFFFAOYSA-N
Related Categories
General description
4′-(Methylthio)acetophenone (4-(Methylthio)acetophenone, p-(methylthio) acetophenone, 4-MTAP) is a sulphur containing organic building block. It is a key intermediate in drug synthesis. Its industrial preparation, via Friedel-Crafts acylation reaction in the presence of aluminium chloride as catalyst and acetyl chloride as acylating agent has been reported. It has been prepared by the acetylation reaction of thioanisole with acetic anhydride in the presence of solid acid catalyst.
Application
4′-(Methylthio)acetophenone may be used for the synthesis of Vioxx (Rofecoxib), an non-steroidal anti-inflammatory drug (NSAID). It may be used in the synthesis of (E)-1-[4-(methylsulfanyl)phenyl]-3-phenylprop-2-en-1-one.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Liquid phase acetylation of thioanisole with acetic anhydride to 4-(methylthio) acetophenone (4-MTAP) using H-beta catalyst.
Sawant DP and Halligudi SB.
Catalysis Communications, 5(11), 659-663 (2004)
(E)-1-[4-(Methylsulfanyl) phenyl]-3-phenylprop-2-en-1-one.
Thiruvalluvar A, et al.
Acta Crystallographica Section E, Structure Reports Online, 64(7), o1263-o1263 (3008)
Experimental and theoretical analysis of Friedel-Crafts acylation of thioanisole to 4-(methylthio) acetophenone using solid acids.
Yadav GD and Bhagat RD.
J. Mol. Catal. A: Chem., 235(1), 98-107 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service