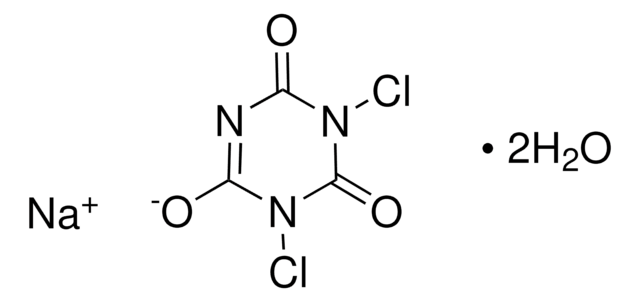
379867
Potassium dicyanoaurate(I)
99.95% trace metals basis
Synonym(s):
Gold (I) potassium cyanide, Potassium gold cyanide
About This Item
Recommended Products
assay
99.95% trace metals basis
form
crystalline
impurities
≤550.0 ppm Trace Metal Analysis
density
3.45 g/mL at 25 °C (lit.)
SMILES string
[K+].N#C[Au-]C#N
InChI
1S/2CN.Au.K/c2*1-2;;/q;;-1;+1
InChI key
OQHPFUBKFKRHKZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- To prepare gold nanowire array electrodes with fast charge transfer ability.
- As a supporting electrolyte for the electrodeposition of gold in organic media.
- To prepare polypyrrole-ClO4 gas sensors with fast response time and high sensitivity to volatile organic compounds.
- In the electrochemical preparation of gold nanoparticle composites.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
supp_hazards
Storage Class
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service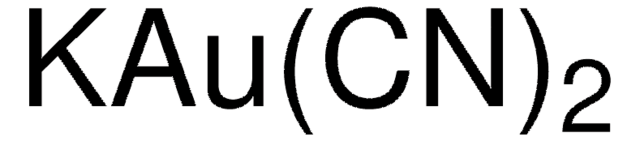

![Cyanide standard solution traceable to SRM from NIST K₂[Zn(CN)₄] in H₂O 1000 mg/l CN Certipur®](/deepweb/assets/sigmaaldrich/product/images/920/032/af45eec3-100b-4996-8eb3-c3942d441bc9/640/af45eec3-100b-4996-8eb3-c3942d441bc9.jpg)