370207
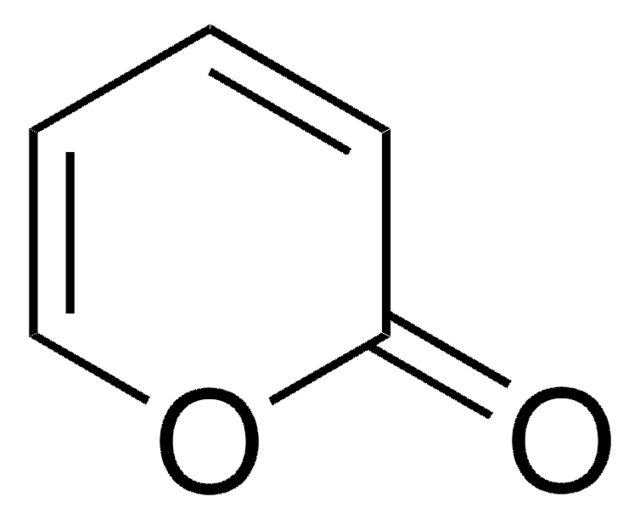
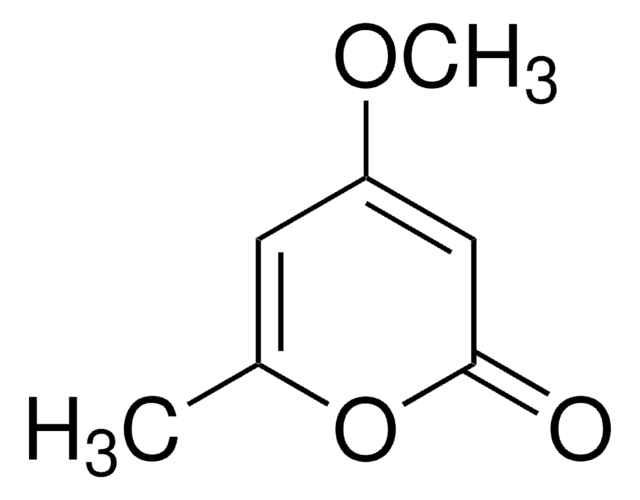
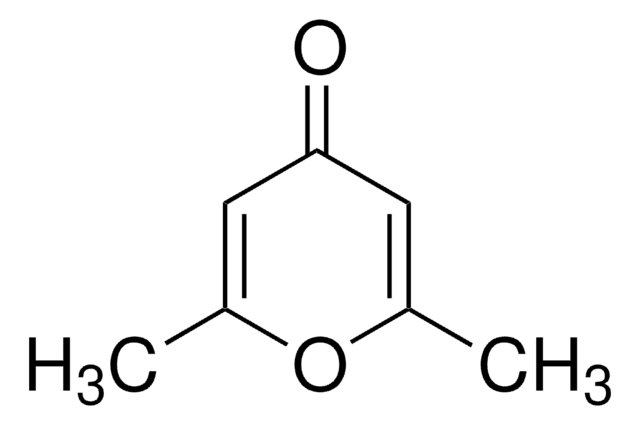
4,6-Dimethyl-α-pyrone
98%
Synonym(s):
4,6-Dimethyl-2H-pyran-2-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H8O2
CAS Number:
Molecular Weight:
124.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
48-50 °C (lit.)
SMILES string
CC1=CC(C)=CC(=O)O1
InChI
1S/C7H8O2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3
InChI key
IXYLIUKQQQXXON-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Related Categories
General description
4,6-Dimethyl-α-pyrone was immobilized in a guanidinium-sulfonate-calixarene (G4C) crystalline network, which upon exposure to ultraviolet irradiation, transforms the entrapped 4,6-dimethyl-α-pyrone into a 4,6-dimethyl-β-lactone. Thin solid films layers of 4,6-dimethyl-α-pyrone on UV irradiation at -190°C were investigated.
Application
4,6-Dimethyl-α-pyrone (Me21) has been used in the preparation of G4C{Me21} via reacting with tetra-p-sulfocalix[4]arene (C) and guanidine hydrochloride (GCl). It may be used in the preparation of host-guest complex G4C{Me21} by reacting with crystalline guanidinium-sulfonate-calixarene (G4C). This complex on UV irradiation afforded 1,3-dimethylcyclobutadiene (Me2CBD).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yves-Marie Legrand et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(36), 10021-10028 (2011-09-08)
Cyclobutadiene (CBD), the smallest cyclic hydrocarbon bearing conjugated double bonds, has long intrigued chemists because of its chemical characteristics. The question of whether the molecule could be prepared at all has been answered, but the parent compound and its unperturbed
Single-crystal X-ray structure of 1,3-dimethylcyclobutadiene by confinement in a crystalline matrix.
Yves-Marie Legrand et al.
Science (New York, N.Y.), 329(5989), 299-302 (2010-07-22)
Cyclobutadiene (CBD), the smallest cyclic hydrocarbon bearing conjugated double bonds, has long intrigued chemists on account of its strained geometry and electronic instability, but the parent compound and its unperturbed derivatives have thus far eluded crystallographic characterization. In this work
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service