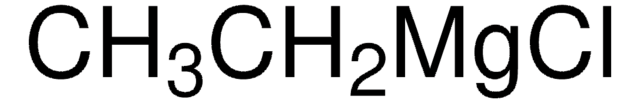345105
Ethylmagnesium bromide solution
1.0 M in tert-butyl methyl ether
Synonym(s):
Bromoethylmagnesium
About This Item
Recommended Products
reaction suitability
reaction type: Grignard Reaction
concentration
1.0 M in tert-butyl methyl ether
density
0.841 g/mL at 25 °C
SMILES string
CC[Mg]Br
InChI
1S/C2H5.BrH.Mg/c1-2;;/h1H2,2H3;1H;/q;;+1/p-1
InChI key
TWTWFMUQSOFTRN-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Application
- Solution Structure and Magnesium Deposition Electrochemistry of Mixing Alkylmagnesium Halide with Magnesium Sulfonyl Amide/Glyme Solution: This research explores the electrochemical behavior of ethylmagnesium bromide in mixed solutions for magnesium deposition applications (Egashira & Hiratsuka, 2016).
- Ethyl magnesium bromide as an efficient anionic initiator for controlled polymerization of ε-caprolactone: The article investigates the use of ethylmagnesium bromide as an initiator for the controlled polymerization of ε-caprolactone (Malinová & Brožek, 2014).
- Room temperature electrodeposition of metallic magnesium from ethylmagnesium bromide in tetrahydrofuran and ionic liquid mixtures: This research focuses on the electrodeposition of metallic magnesium using ethylmagnesium bromide in various solvent mixtures (Chen et al., 2015).
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Water-react 1
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 1
flash_point_f
-29.2 °F - closed cup
flash_point_c
-34 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service