305782
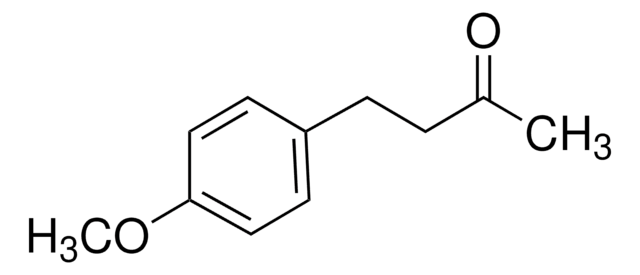
4-(3-Oxobutyl)phenyl acetate
≥96%
Synonym(s):
4-(4-Acetoxyphenyl)-2-butanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CO2C6H4CH2CH2COCH3
CAS Number:
Molecular Weight:
206.24
Beilstein/REAXYS Number:
1961620
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥96%
form
liquid
refractive index
n20/D 1.509 (lit.)
bp
123-124 °C/0.2 mmHg (lit.)
density
1.099 g/mL at 25 °C (lit.)
functional group
ester
ketone
SMILES string
CC(=O)CCc1ccc(OC(C)=O)cc1
InChI
1S/C12H14O3/c1-9(13)3-4-11-5-7-12(8-6-11)15-10(2)14/h5-8H,3-4H2,1-2H3
InChI key
UMIKWXDGXDJQJK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(3-Oxobutyl)phenyl acetate (4-(4-acetoxyphenyl) -2-butanone) is a standard melon fly attractant.
Application
4-(3-Oxobutyl)phenyl acetate was used as attractant for detection programs aimed at melon fly and other cuelure-responding Bactrocera fruit flies.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Eric B Jang et al.
Journal of economic entomology, 100(4), 1124-1128 (2007-09-14)
Field-trapping evaluations of the new male attractant, formic acid 4-(3-oxobutyl) phenyl ester (raspberry ketone formate [RKF]) were conducted in Hawaii with wild populations of melon flies, Bactrocera cucurbitae Coquillett (Diptera: Tephritidae), to determine its activity in the field and to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service