All Photos(1)
About This Item
Empirical Formula (Hill Notation):
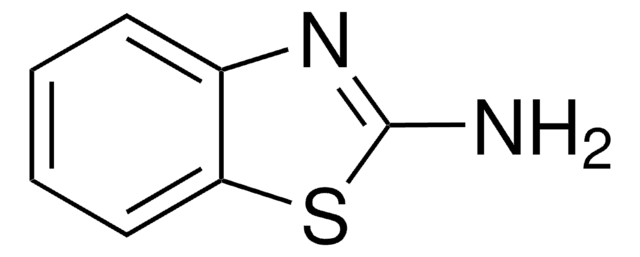
C8H8N2S
CAS Number:
Molecular Weight:
164.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
mp
140-142 °C (lit.)
SMILES string
Cc1ccc2nc(N)sc2c1
InChI
1S/C8H8N2S/c1-5-2-3-6-7(4-5)11-8(9)10-6/h2-4H,1H3,(H2,9,10)
InChI key
DZWTXWPRWRLHIL-UHFFFAOYSA-N
General description
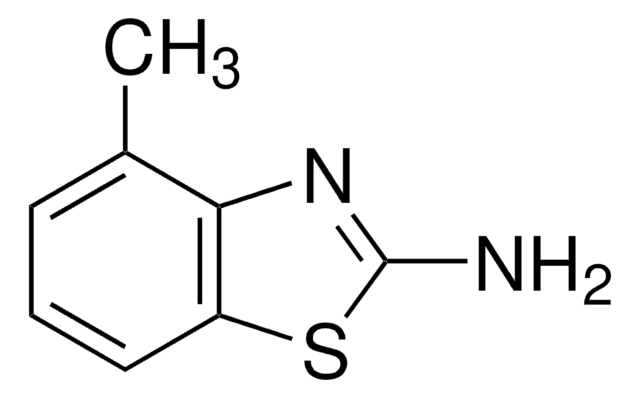
The anti-tetanus activity of 2-amino-6-methylbenzothiazole, a muscle relactant, was studied.
Application
2-Amino-6-methylbenzothiazole was used in the preparation of 2-[(6-methyl-1,3-benzothiazol-2-yl)amino]-N-[2-(substituted phenyl/furan-2-yl)-4-oxo-1,3-thiazolidin-3-yl]nicotinamides.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R A WEBSTER
British journal of pharmacology and chemotherapy, 17, 507-518 (1961-12-01)
The anti-tetanus activity of a number of phenothiazine derivatives and other centrally acting muscle relaxants, such as mephenesin, dicyclopropyl ketoxime, 2-amino-6-methylbenzothiazole and meprobamate, has been determined in rabbits with experimental local tetanus. Structure-activity relationships were obtained for the phenothiazine derivatives
Navin B Patel et al.
Scientia pharmaceutica, 78(4), 753-765 (2010-12-24)
The title compounds 6aâj, 2-[(6-methyl-1,3-benzothiazol-2-yl)amino]-N-[2-(substituted phenyl/furan-2-yl)-4-oxo-1,3-thiazolidin-3-yl]nicotinamides, were prepared from 2-chloropyridine-3-carboxylic acid (1) and 2-amino-6-methyl-benzothiazole (2) by known methods. All the compounds have been established by IR, (1)H NMR, (13)C NMR and elemental analyses. The in vitro antimicrobial screening of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service