230995
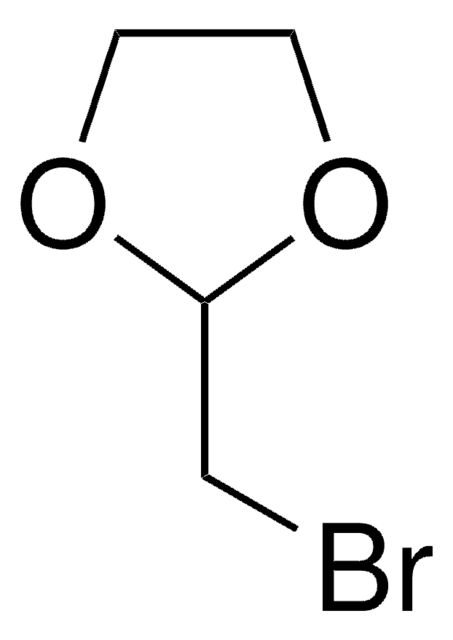
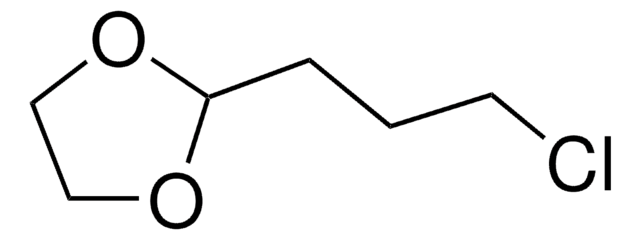
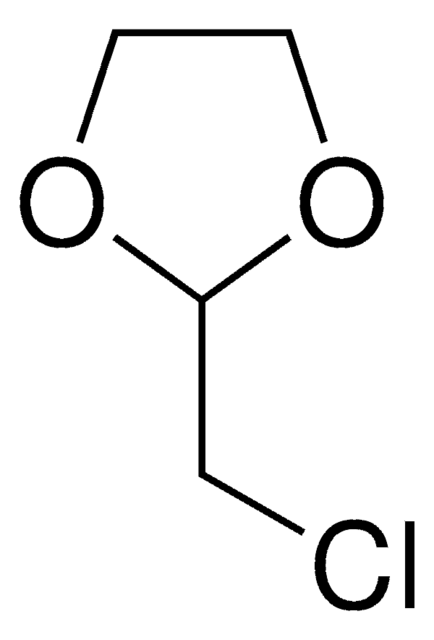
2-(2-Bromoethyl)-1,3-dioxolane
96%
Synonym(s):
3-Bromopropionaldehyde ethylene acetal
About This Item
Recommended Products
Quality Level
assay
96%
form
liquid
contains
sodium bicarbonate as stabilizer
refractive index
n20/D 1.479 (lit.)
bp
68-70 °C/8 mmHg (lit.)
density
1.542 g/mL at 25 °C (lit.)
functional group
bromo
ether
storage temp.
2-8°C
SMILES string
BrCCC1OCCO1
InChI
1S/C5H9BrO2/c6-2-1-5-7-3-4-8-5/h5H,1-4H2
InChI key
GGZQLTVZPOGLCC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-(2-Bromoethyl)-1,3-dioxolane is a cyclic ether derivative with a dioxolane ring, used as a building block in organic synthesis and polymer industry.
Application
- as starting reagent for the synthesis of (5Z,9Z)-5,9-hexadecadienoic acid, (5Z,9Z)-5,9-nonadecadienoic acid and (5Z,9Z)-5,9-eicosadienoic acid
- in synthesis of 1-deoxy-castanospermine and 1-deoxy-8a-epi-castanospermine
- in asymmetric total synthesis of both enantiomers of marine mollusk metabolite pulo′upone via Evans′ asymmetric Diels-Alder reaction
- as alkylating agent for amines, dithianes and carboximides
- with eynamides and sodium azide in a "one-pot" synthesis of triazoles
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
flash_point_f
149.0 °F - closed cup
flash_point_c
65 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service