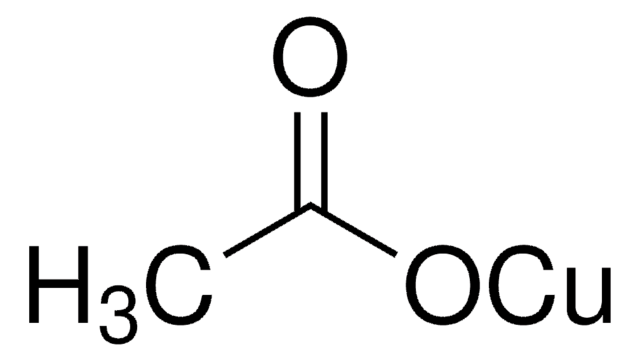
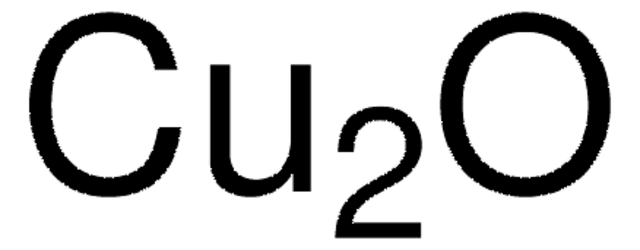229628
Copper(I) chloride
≥99.995% trace metals basis
Synonym(s):
Copper monochloride, Cuprous chloride
About This Item
Recommended Products
vapor pressure
1.3 mmHg ( 546 °C)
Quality Level
assay
≥99.995% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: copper
technique(s)
mass spectrometry (MS): suitable
impurities
≤50.0 ppm Trace Rare Earth Analysis
bp
1490 °C (lit.)
mp
430 °C (lit.)
solubility
slightly soluble 0.47 g/L at 20 °C
application(s)
battery manufacturing
SMILES string
Cl[Cu]
InChI
1S/ClH.Cu/h1H;/q;+1/p-1
InChI key
OXBLHERUFWYNTN-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
Shows unique character as an initiator of radical reactions such as the hydrostannation of α,β-unsaturated ketones.
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
Thermoelectric Performance of Perovskite-type Oxide Materials
Spectral conversion for solar cells is an emerging concept in the field of photovoltaics, and it has the potential to increase significantly the efficiency of solar cells. Lanthanide ions are ideal candidates for spectral conversion, due to their high luminescence efficiencies and rich energy level structure that allows for great flexibility in the upconversion and downconversion of photons in a wide spectral region (NIR-VIS-UV).
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Protocols
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service