All Photos(5)
About This Item
Empirical Formula (Hill Notation):
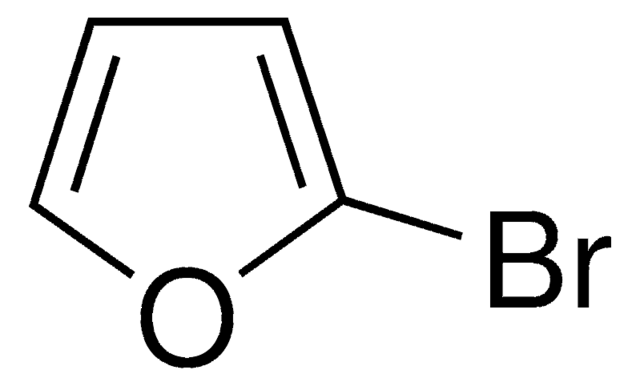
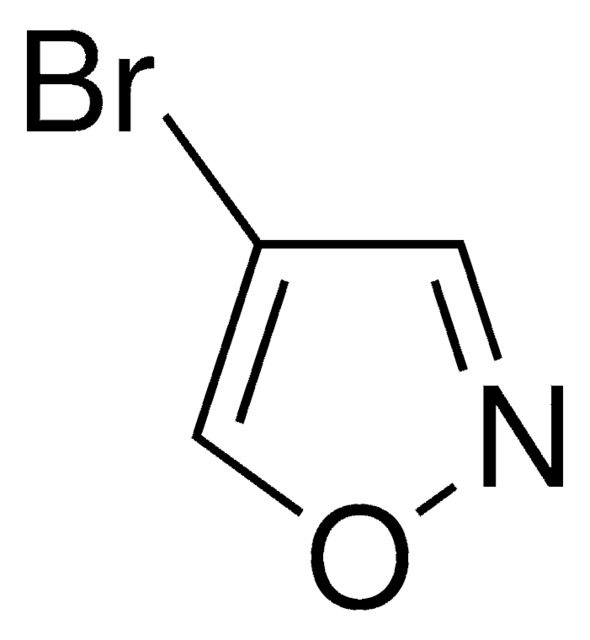
C4H3BrO
CAS Number:
Molecular Weight:
146.97
Beilstein/REAXYS Number:
105337
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
liquid
refractive index
n20/D 1.496 (lit.)
bp
103 °C (lit.)
density
1.635 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
−20°C
SMILES string
Brc1ccoc1
InChI
1S/C4H3BrO/c5-4-1-2-6-3-4/h1-3H
InChI key
LXWLEQZDXOQZGW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Bromofuran was used in the preparation of 2-substituted 3-furfurals. It was also used in the synthesis of 5,6-dehydronorcantharidins.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
37.4 °F - closed cup
flash_point_c
3 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ann Rowley Kelly et al.
Journal of the American Chemical Society, 130(12), 4097-4104 (2008-03-05)
Furans and pyrroles are important synthons in chemical synthesis and are commonly found in natural products, pharmaceutical agents, and materials. Introduced herein are three methods to prepare 2-substituted 3-furfurals starting from 3-furfural, 3-bromofuran, and 3-vinylfurans. Addition of a variety of
Adam McCluskey et al.
Bioorganic chemistry, 31(1), 68-79 (2003-04-17)
Diels-Alder addition of furans (furan, furfuryl alcohol, and 3-bromofuran) to maelic anhydride yields three distinct 5,6-dehydronorcantharidins. Hydrogenation of (4,10-dioxatricyclo[5.2.1.0]decane-3,5-dione) (4a), in dry ethanol affords the monoester (7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic aid monoethyl ester) (6). Subsequent transesterification affords a series of monoesters (7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service