157864
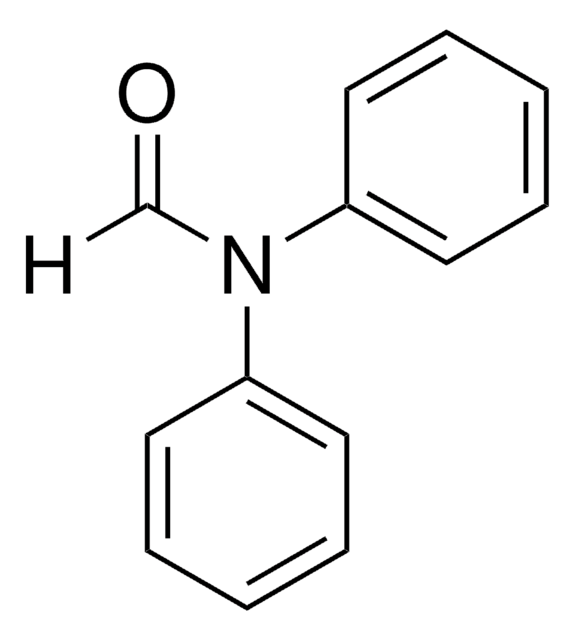
1-Acetylimidazole
98%
Synonym(s):
1-(1H-Imidazol-1-yl)ethan-1-one, 1-(1H-Imidazol-1-yl)ethanone, 1-Acetyl-1H-imidazole, N-Acetylimidazole
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C5H6N2O
CAS Number:
Molecular Weight:
110.11
Beilstein/REAXYS Number:
108425
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
99-105 °C (lit.)
solubility
water: soluble 50 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
CC(=O)n1ccnc1
InChI
1S/C5H6N2O/c1-5(8)7-3-2-6-4-7/h2-4H,1H3
InChI key
VIHYIVKEECZGOU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Rate of solvolysis of 1-acetylimidazole in acid solutions was evaluated. Reaction of 1-acetylimidazole with orthophosphate and adenosine-5′-phosphate has been reported.
Application
1-Acetylimidazole was used as acetylation reagent for amino groups. It was also employed for acetylation of histones.
Relatively specific reagent for tyrosyl residue acetylation. Reagent used in the synthesis of annulated imidazole derivatives.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I Wells et al.
Biochemistry, 31(40), 9520-9525 (1992-10-13)
Treatment of prostaglandin endoperoxide (PGH) synthase apoprotein with a 100- or 1000-fold excess of N-acetylimidazole (NAI) led to time-dependent inactivation of both cyclooxygenase and peroxide activities. Reconstitution of apoprotein with heme prior to incubation with NAI substantially protected the enzyme
Chemische Berichte, 125, 1939-1939 (1992)
The Solvolysis of 1-Acetylimidazole in Concentrated Acid and Salt Solutions.
Marburg S and Jencks WP.
Journal of the American Chemical Society, 84(2), 232-239 (1962)
Variation of the nuclear, subnuclear and chromosomal flavanol deposition in hemlock and rye.
Feucht W, et al.
International Journal of Molecular Sciences, 8(7), 635-650 (2007)
Hong Xu et al.
Protein and peptide letters, 10(5), 503-509 (2003-10-17)
Cinnamomin is a type II ribosome-inactivating protein (RIP) and its A-chain (CTA) is a RNA N-glycosidase. It is observed that modification of tyrosine residues by N-acetylimidazole (N-AI) causes almost complete loss of CTA activity. Adenine partially protects tyrosine residues from
Global Trade Item Number
| SKU | GTIN |
|---|---|
| S752665-1EA | |
| 157864-100G | 4061833554364 |
| 157864-1KG | 4061826680193 |
| 157864-25G | 4061838743664 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service