All Photos(1)
About This Item
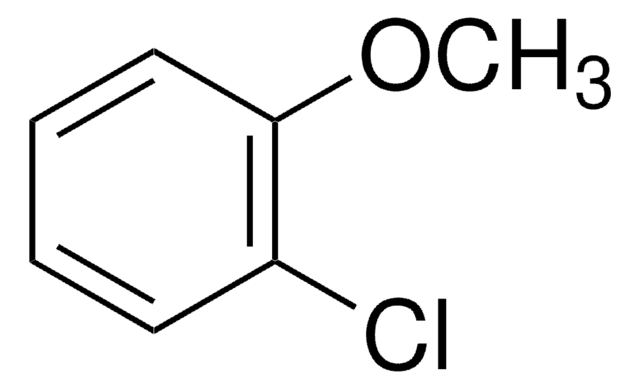
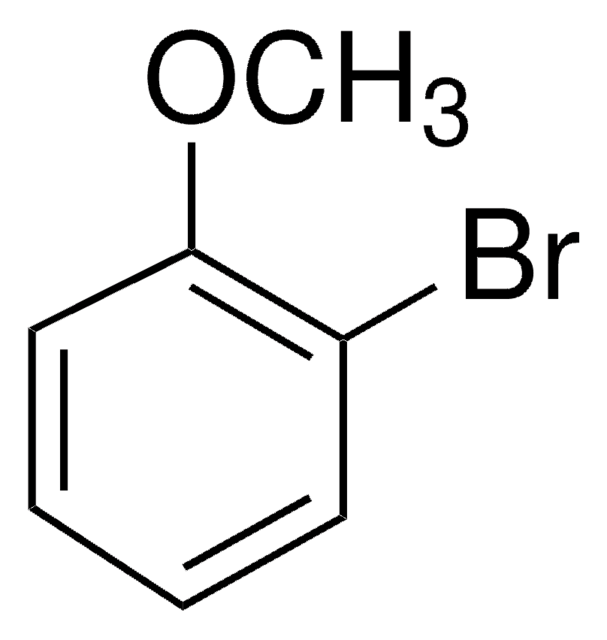
Linear Formula:
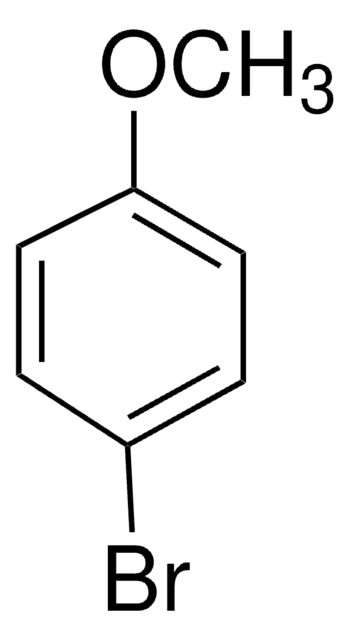
ClC6H4OCH3
CAS Number:
Molecular Weight:
142.58
Beilstein/REAXYS Number:
2041497
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.536 (lit.)
bp
193 °C (lit.)
density
1.164 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
COc1cccc(Cl)c1
InChI
1S/C7H7ClO/c1-9-7-4-2-3-6(8)5-7/h2-5H,1H3
InChI key
YUKILTJWFRTXGB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
One-step preparation of 3-chloroanisole from the corresponding 3-substituted nitrobenzene has been reported.
Application
3-Chloroanisole was employed as starting reagent in the regioselective synthesis of 4- and 7-alkoxyindoles. It was also employed as electrolyte additive for the overcharging protection of Li-ion cell.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
163.4 °F - closed cup
flash_point_c
73 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
3-Chloroanisole for overcharge protection of a Li-ion cell.
Lee Y-G and Cho J.
Electrochimica Acta, 52(25), 7404-7408 (2007)
Roberto Sanz et al.
The Journal of organic chemistry, 72(14), 5113-5118 (2007-06-15)
An efficient and regioselective synthesis of 4- and 7-alkoxyindoles has been developed from commercially available starting materials such as 3-halophenols and 3-chloroanisole. Directed ortho-metalation followed by two palladium-catalyzed processes, a Sonogashira coupling and a tandem amination/cyclization reaction, allows the synthesis
One-step preparation of some 3-substituted anisoles.
Zilberman J.
Organic Process Research & Development, 7(3), 303-305 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service