All Photos(3)
About This Item
Empirical Formula (Hill Notation):
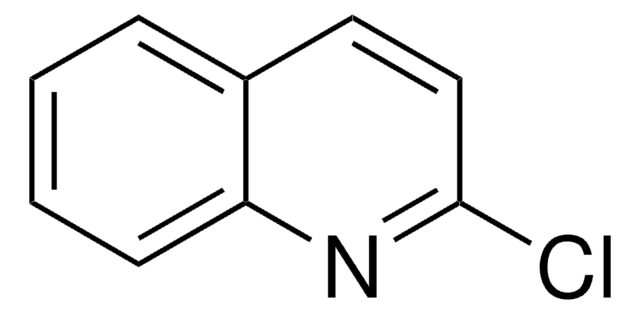
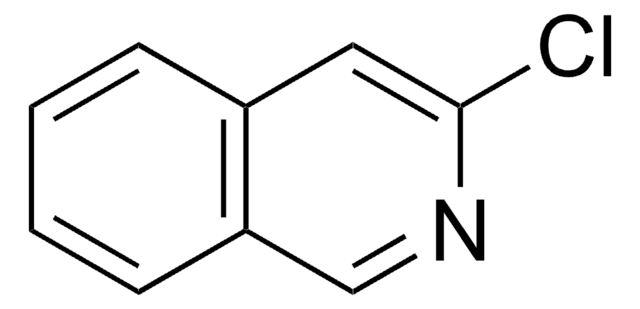
C9H6ClN
CAS Number:
Molecular Weight:
163.60
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
bp
274-275 °C/768 mmHg (lit.)
mp
31-36 °C (lit.)
functional group
chloro
SMILES string
Clc1nccc2ccccc12
InChI
1S/C9H6ClN/c10-9-8-4-2-1-3-7(8)5-6-11-9/h1-6H
InChI key
MSQCQINLJMEVNJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
The product has been used in a Mn-catalyzed cross-coupling with aryl- and alkylmagnesium halides. It has also been used in a Pd-catalyzed cross-coupling with heteroaryl boronic acids and esters. Furthermore, it has been used in a homocoupling reaction to yield bis-isoquinoline, each enantiomer of which might be very useful as a chiral ligand for asymmetric synthesis.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Homocoupling of aryl iodides and bromides using a palladium/indium bimetallic system.
Chang, Yu Mi, et al.
Synthetic Communications, 35(13), 1851-1857 (2005)
Synlett, 247-247 (2007)
Kelvin Billingsley et al.
Journal of the American Chemical Society, 129(11), 3358-3366 (2007-03-01)
A highly active and efficient catalyst system derived from a palladium precatalyst and monophosphine ligands 1 or 2 for the Suzuki-Miyaura cross-coupling reaction of heteroaryl boronic acids and esters has been developed. This method allows for the preparation of a
Nelson R Vinueza et al.
Physical chemistry chemical physics : PCCP, 20(33), 21567-21572 (2018-08-11)
Two previously unreported isomeric biradicals with a 1,4-radical topology, the 1,5-didehydroisoquinolinium cation and the 4,8-didehydroisoquinolinium cation, and an additional, previously reported isomer, the 4,5-didehydroisoquinolinium cation, were studied to examine the importance of the exact location of the radical sites on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service