129216
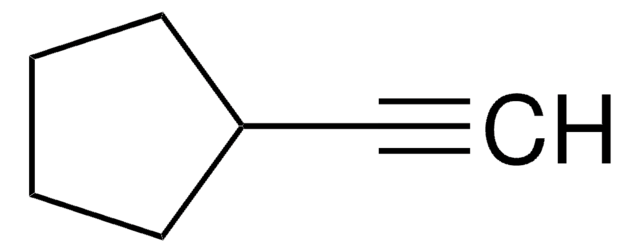
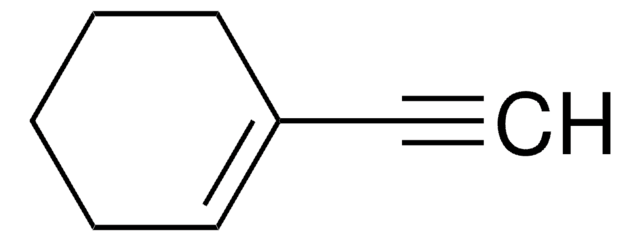
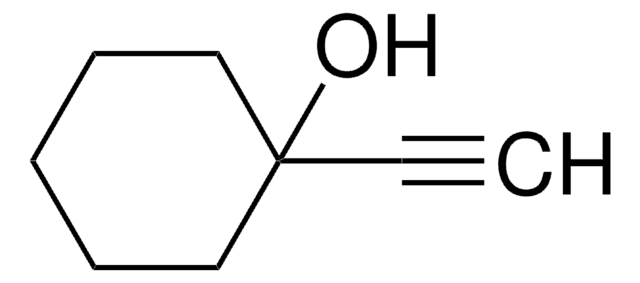
Cyclohexylacetylene
98%
Synonym(s):
Ethynylcyclohexane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H11C≡CH
CAS Number:
Molecular Weight:
108.18
Beilstein/REAXYS Number:
1815535
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
98%
form
liquid
refractive index
n20/D 1.4540 (lit.)
bp
130-132 °C (lit.)
density
0.828 g/mL at 25 °C (lit.)
SMILES string
C#CC1CCCCC1
InChI
1S/C8H12/c1-2-8-6-4-3-5-7-8/h1,8H,3-7H2
InChI key
SSDZYLQUYMOSAK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Cyclohexylacetylene (Ethynylcyclohexane) mediates NADPH-dependent loss of cytochrome P-450 on incubation with hepatic microsomes.
Application
Cyclohexylacetylene (Ethynylcyclohexane) was used in the preparation of [Os[(E)-CH=CHR](=C=C=CPh2)(CH3CN)2(P(i)Pr3)2]BF4 (R = Ph, Cy).
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
64.4 °F - closed cup
flash_point_c
18 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Self-catalyzed inactivation of hepatic cytochrome P-450 by ethynyl substrates.
P R Ortiz de Montellano et al.
The Journal of biological chemistry, 255(12), 5578-5585 (1980-06-25)
Tamara Bolaño et al.
Journal of the American Chemical Society, 128(12), 3965-3973 (2006-03-23)
Treatment in acetonitrile at -30 C of the hydride-alkenylcarbyne complex [OsH([triple bond]CCH=CPh2)(CH3CN)2(P(i)Pr3)2][BF4]2 (1) with (t)BuOK produces the selective deprotonation of the alkenyl substituent of the carbyne and the formation of the bis-solvento hydride-allenylidene derivative [OsH(=C=C=CPh2)(CH3CN)2(P(i)Pr3)2]BF4 (2), which under carbon monoxide
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service