127485
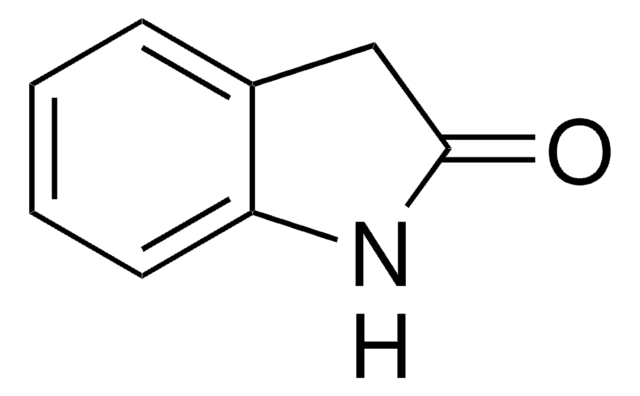
5-Chloro-2-oxindole
98%
Synonym(s):
5-Chlorooxindole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H6ClNO
CAS Number:
Molecular Weight:
167.59
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
mp
194-197 °C (lit.)
solubility
methanol: soluble 2.5 mL, clear
functional group
chloro
SMILES string
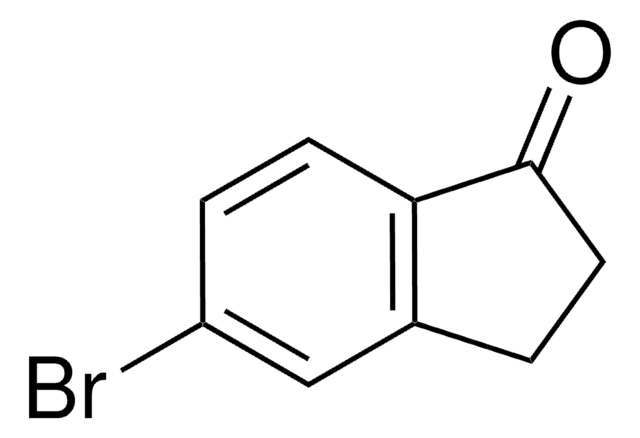
Clc1ccc2NC(=O)Cc2c1
InChI
1S/C8H6ClNO/c9-6-1-2-7-5(3-6)4-8(11)10-7/h1-3H,4H2,(H,10,11)
InChI key
WWJLCYHYLZZXBE-UHFFFAOYSA-N
Related Categories
General description
5-Chloro-2-oxindole (5-Chlorooxindole) is a starting material for tenidap sodium, a pharmaceutical drug candidate.
Application
5-Chloro-2-oxindole was used for the quantitation of 5-chloro-2-oxindole, concomitantly with all of its potential positional isomers using a single, highly specific, normal-phase chromatographic system.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Repr. 2 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S T Colgan et al.
Journal of pharmaceutical and biomedical analysis, 14(7), 825-833 (1996-05-01)
5-Chlorooxindole (5-CO) is a starting material for tenidap sodium, a pharmaceutical drug candidate produced by Pfizer. To insure potency and purity of the drug substance, it is necessary to demonstrate that commercial supplies of 5-CO are free from elevated levels
Boris Letribot et al.
Molecules (Basel, Switzerland), 23(6) (2018-06-13)
Alkylidene oxindoles are important functional moieties and building blocks in pharmaceutical and synthetic chemistry. Our interest in biologically active compounds focused our studies on the synthesis of novel oxindoles, bearing on the exocyclic double bond at the C8, CN, and
Ayman M Saleh et al.
Molecules (Basel, Switzerland), 19(9), 13076-13092 (2014-08-27)
A selected set of substituted pyridone-annelated isoindigos 3a-f has been synthesized via interaction of 5- and 6-substituted oxindoles 2a-f with 6-ethyl-1,2,9-trioxopyrrolo[3,2-f]quinoline-8-carboxylic acid (1) in acetic acid at reflux. Among these isoindigos, the 5'-chloro and 5'-bromo derivatives 3b and 3d show
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service