122068
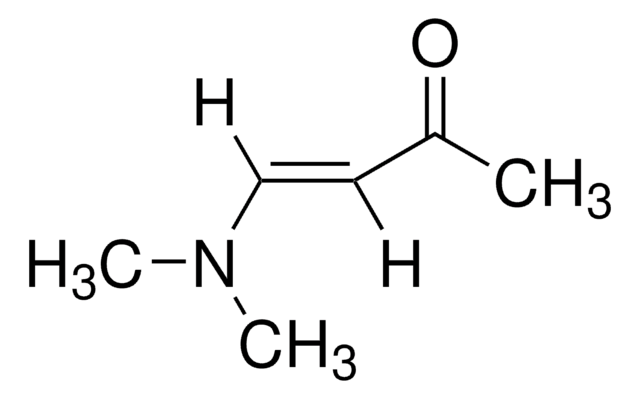
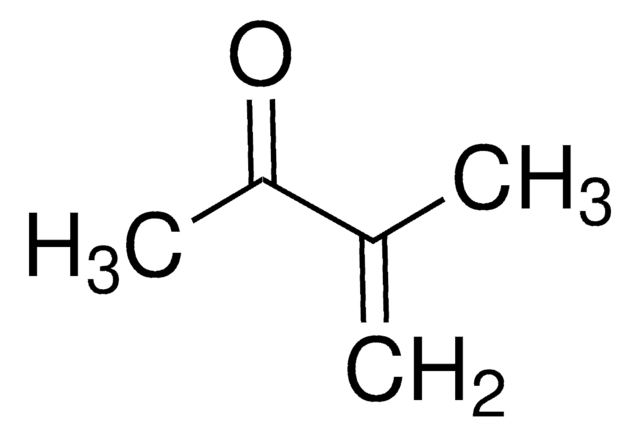
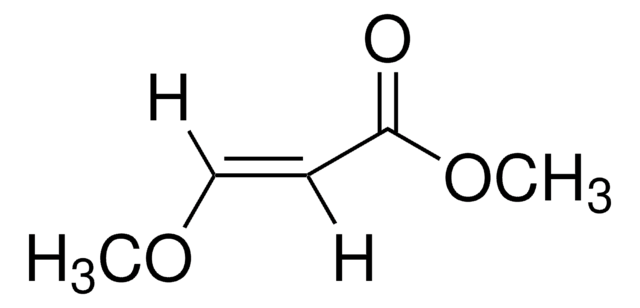
trans-4-Methoxy-3-buten-2-one
technical grade, 90%
Synonym(s):
(E)-4-Methoxy-3-buten-2-one
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OCH=CHCOCH3
CAS Number:
Molecular Weight:
100.12
Beilstein/REAXYS Number:
2070991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
assay
90%
refractive index
n20/D 1.468 (lit.)
bp
200 °C (lit.)
density
0.982 g/mL at 25 °C (lit.)
functional group
ether
ketone
storage temp.
2-8°C
SMILES string
[H]\C(OC)=C(\[H])C(C)=O
InChI
1S/C5H8O2/c1-5(6)3-4-7-2/h3-4H,1-2H3/b4-3+
InChI key
VLLHEPHWWIDUSS-ONEGZZNKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
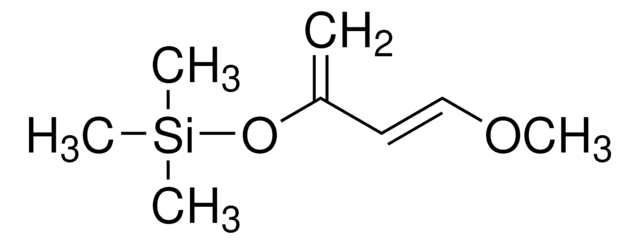
trans-4-Methoxy-3-buten-2-one acts as substrate and undergoes zinc triflate-catalyzed Mukaiyama-Michael reaction with 3-TBSO-substituted vinyldiazoacetate to yield functionalized 3-keto-2-diazoalkanoates.
Application
trans-4-Methoxy-3-buten-2-one was used as starting reagent for enantioselective total synthesis of (-)-epibatidine.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
145.4 °F - closed cup
flash_point_c
63 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bifunctional thiourea-catalyzed enantioselective double Michael reaction of ?, d-unsaturated ?-ketoester to nitroalkene: asymmetric synthesis of (-)-epibatidine.
Hoashi Y, et al.
Tetrahedron Letters, 45(50), 9185-9188 (2004)
Yu Liu et al.
Organic letters, 12(19), 4304-4307 (2010-09-03)
Enedione-diazoesters formed from 3-TBSO-2-diazo-3-butenoates undergo base-catalyzed pericyclization that with dinitrogen extrusion and methyl migration provide a novel and efficient route to 2-carboalkoxyresorcinols. Intercepting the intermediate enolate anion with methyl vinyl ketone leads to the corresponding 4-substituted 2-carboalkoxyresorcinol and suggests generalization
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service