推薦產品
等級
pharmaceutical primary standard
API 家族
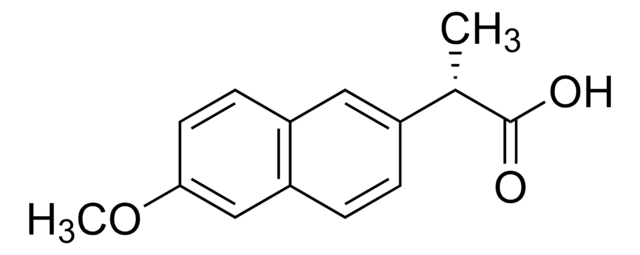
naproxen
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
[Na+].COc1ccc2cc(ccc2c1)[C@H](C)C([O-])=O
InChI
1S/C14H14O3.Na/c1-9(14(15)16)10-3-4-12-8-13(17-2)6-5-11(12)7-10;/h3-9H,1-2H3,(H,15,16);/q;+1/p-1/t9-;/m0./s1
InChI 密鑰
CDBRNDSHEYLDJV-FVGYRXGTSA-M
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
尋找類似的產品? 前往 產品比較指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Naproxen sodium USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Naproxen Sodium and Pseudoephedrine Hydrochloride Extended-Release Tablets
- Naproxen Sodium Tablets
生化/生理作用
环加氧酶(前列腺素 H 合成酶 1 和 2)抑制剂。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Repr. 1A
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
分析證明 (COA)
輸入產品批次/批號來搜索 分析證明 (COA)。在產品’s標籤上找到批次和批號,寫有 ‘Lot’或‘Batch’.。
Arın Gül Dal et al.
Journal of analytical methods in chemistry, 2014, 352698-352698 (2014-10-09)
Simple and rapid capillary zone electrophoretic method was developed and validated in this study for the determination of piroxicam in tablets. The separation of piroxicam was conducted in a fused-silica capillary by using 10 mM borate buffer (pH 9.0) containing 10%
Matteo Morotti et al.
European journal of obstetrics, gynecology, and reproductive biology, 179, 63-68 (2014-06-27)
Evaluate patient satisfaction at 6-month treatment in women with symptomatic rectovaginal endometriosis and migraine without aura with (progestogen-only contraceptive pill, POP versus sequential combined oral contraceptives, COC) STUDY DESIGN: A patient preference trial including 144 women (82 in the group
Jason A Miranda et al.
PloS one, 9(8), e106108-e106108 (2014-08-27)
Sensory processing in the spinal cord during disease states can reveal mechanisms for novel treatments, yet very little is known about pain processing at this level in the most commonly used animal models of articular pain. Here we report a
Tarjinder Sahota et al.
Toxicology and applied pharmacology, 278(3), 209-219 (2014-03-29)
The assessment of safety in traditional toxicology protocols relies on evidence arising from observed adverse events (AEs) in animals and on establishing their correlation with different measures of drug exposure (e.g., Cmax and AUC). Such correlations, however, ignore the role
Jennifer Y Xie et al.
Pain, 155(8), 1659-1666 (2014-05-28)
Preclinical assessment of pain has increasingly explored operant methods that may allow behavioral assessment of ongoing pain. In animals with incisional injury, peripheral nerve block produces conditioned place preference (CPP) and activates the mesolimbic dopaminergic reward pathway. We hypothesized that
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務