推薦產品
等級
pharmaceutical primary standard
API 家族
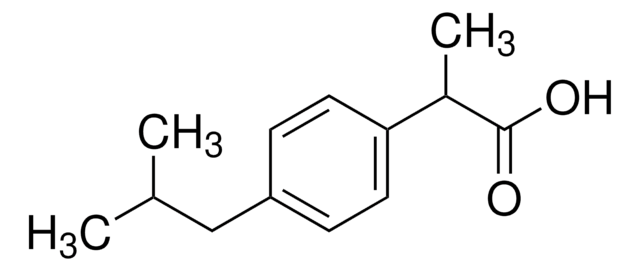
ibuprofen
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
SMILES 字串
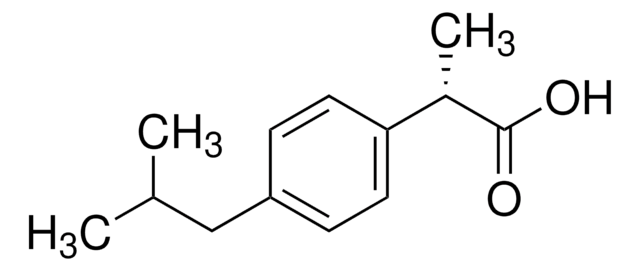
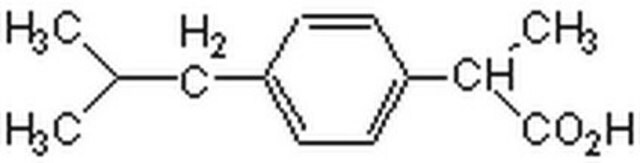
CC(C)Cc1ccc(cc1)C(C)C(O)=O
InChI
1S/C13H18O2/c1-9(2)8-11-4-6-12(7-5-11)10(3)13(14)15/h4-7,9-10H,8H2,1-3H3,(H,14,15)
InChI 密鑰
HEFNNWSXXWATRW-UHFFFAOYSA-N
基因資訊
human ... PTGS1(5742) , PTGS2(5743)
尋找類似的產品? 前往 產品比較指南
相關類別
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Ibuprofen USP reference standard suitable for use in specified USP compendial tests and assays.
Also used to prepare standard preparation, standard and standard stock solution for performance tests, assay, identification, and impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard preparation, standard and standard stock solution for performance tests, assay, identification, and impurity analysis according to the given below monographs of United States Pharmacopeia (USP):
- Diphenhydramine Hydrochloride and Ibuprofen Capsules
- Ibuprofen and Pseudoephedrine Hydrochloride Tablets
生化/生理作用
环加氧酶 (COX) 抑制剂,对 COX-1 的抑制活性大于对 COX-2 的抑制活性。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
客戶也查看了
Osteoarthritis of the knee and hip. Part II: therapy with ibuprofen and a review of clinical trials.
Aleem Adatia et al.
The Journal of pharmacy and pharmacology, 64(5), 626-636 (2012-04-05)
We review the pharmacological properties and clinical evidence pertaining to the efficacy of ibuprofen as a first-line treatment in hip and knee osteoarthritis (OA). In the context of our previous paper's exploration of the aetiology and pathogenesis of OA as
Roy Rabbie et al.
The Cochrane database of systematic reviews, 4(4), CD008039-CD008039 (2013-05-02)
This is an updated version of the original review published in Issue 10, 2010 (Rabbie 2010). Migraine is a common, disabling condition and a burden for the individual, health services and society. Many sufferers do not seek professional help, relying
Yexuan Mao et al.
Talanta, 132, 894-901 (2014-12-06)
The fundamental studies for the binding events of protein and its partner are crucial in drug development. In this study, a novel technology named microscale thermophoresis (MST) was applied in the investigation of molecular interaction between an organic dye fluorescein
Sheena Derry et al.
The Cochrane database of systematic reviews, 10(10), CD007550-CD007550 (2013-10-24)
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews Issue 3, 2009 on single dose oral dexibuprofen (S(+)-ibuprofen) for acute postoperative pain in adults.Dexibuprofen is a non-steroidal anti-inflammatory drug (NSAID) licensed for
Roland Neumann et al.
Neonatology, 102(1), 9-15 (2012-03-15)
Pharmacological closure of patent ductus arteriosus (PDA) is commonly achieved by intravenous (IV) administration of ibuprofen or indomethacin. Occasionally, oral ibuprofen is used for PDA treatment although its efficacy and safety are unclear. To systematically review randomized and quasi-randomized trials
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務