Z2001
Zonisamide sodium salt
≥98% (HPLC), powder
同義詞:
1,2-Benzisoxazole-3-methanesulfonamide sodium-potassium salt, Aleviatin sodium-potassium salt, Exceglan sodium-potassium salt, Excegram sodium-potassium salt
登入查看組織和合約定價
全部照片(1)
About This Item
經驗公式(希爾表示法):
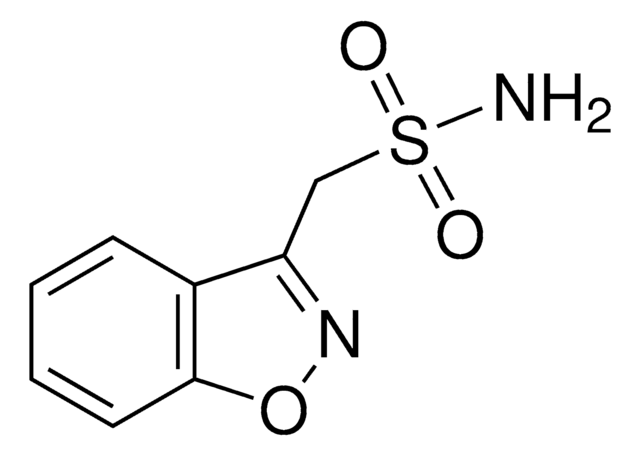
C8H7N2NaO3S
CAS號碼:
分子量::
234.21
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推薦產品
品質等級
化驗
≥98% (HPLC)
形狀
powder
顏色
white to beige
溶解度
H2O: >5 mg/mL
起源
Eisai
SMILES 字串
[Na]NS(=O)(=O)Cc1noc2ccccc12
InChI
1S/C8H7N2O3S.Na/c9-14(11,12)5-7-6-3-1-2-4-8(6)13-10-7;/h1-4H,5H2,(H-,9,11,12);/q-1;+1
InChI 密鑰
ZVBIRPKGWOVBLG-UHFFFAOYSA-N
基因資訊
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
生化/生理作用
Zonisamide sodium salt is an anti-epileptic and also scavenges nitric oxide (NO).
Zonisamide sodium salt is an anti-epileptic. It is effective in various animal epilepsy models and humans with both partial and generalized epileptic seizures. Zonisamide sodium salt also scavenges nitric oxide (NO).
特點和優勢
This compound was developed by Eisai. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
客戶也查看了
M E Choudhury et al.
European journal of pharmacology, 689(1-3), 72-80 (2012-06-05)
Zonisamide has been proven as an effective drug for the recovery of degenerating dopaminergic neurons in the animal models of Parkinson's disease. However, several lines of evidence have questioned the neuroprotective capacity of zonisamide in animal models of Parkinson's disease.
Michel Baulac et al.
The Lancet. Neurology, 11(7), 579-588 (2012-06-12)
Additional options are needed for monotherapy treatment of adults newly diagnosed with partial epilepsy. This trial compares the efficacy and tolerability of once-daily zonisamide with twice-daily controlled-release carbamazepine monotherapy for such patients. In this phase 3, randomised, double-blind, parallel-group, non-inferiority
S Dupont et al.
Acta neurologica Scandinavica. Supplementum, (194)(194), 29-35 (2012-11-01)
Zonisamide is currently licensed in Europe and the USA for the adjunctive treatment of partial seizures (with or without secondary generalization) in adults, based on the results of four pivotal, randomized, double-blind, placebo-controlled trials. It is also licensed in Europe
Andrea Romigi et al.
Epilepsy & behavior : E&B, 26(2), 170-174 (2013-01-15)
The purpose of this study was to evaluate the effects of zonisamide (ZNS) as adjunctive therapy on sleep-wake cycle and daytime somnolence in adult patients affected by focal epilepsy. Thirteen patients affected by focal epilepsy were recruited to undergo a
Kouji Fukuyama et al.
Pharmaceuticals (Basel, Switzerland), 13(6) (2020-06-11)
Recent studies using the genetic partial epilepsy model have demonstrated that hyperfunction of astroglial hemichannels contributes to pathomechanism of epileptic seizure. Therefore, to explore the novel anticonvulsive mechanisms, the present study determined the effects of voltage-dependent Na+ channel (VDSC)-inhibiting anticonvulsants
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務