全部照片(1)
About This Item
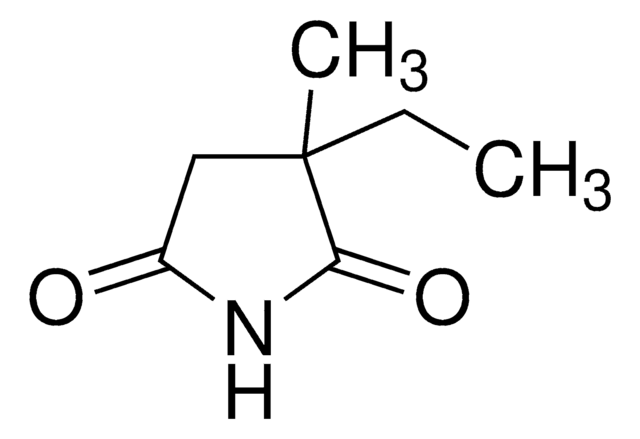
經驗公式(希爾表示法):
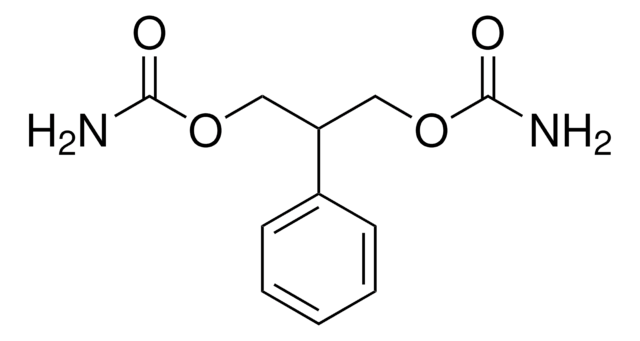
C11H14N2O4
CAS號碼:
分子量::
238.24
EC號碼:
MDL號碼:
分類程式碼代碼:
12352200
PubChem物質ID:
NACRES:
NA.77
推薦產品
品質等級
溶解度
alcohol: soluble
SMILES 字串
NC(=O)OCC(COC(N)=O)c1ccccc1
InChI
1S/C11H14N2O4/c12-10(14)16-6-9(7-17-11(13)15)8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H2,12,14)(H2,13,15)
InChI 密鑰
WKGXYQFOCVYPAC-UHFFFAOYSA-N
基因資訊
human ... GRIN1(2902) , GRIN2A(2903) , GRIN2B(2904) , GRIN2C(2905) , GRIN2D(2906) , GRIN3A(116443) , GRIN3B(116444)
尋找類似的產品? 前往 產品比較指南
生化/生理作用
Anticonvulsant agent that is an allosteric antagonist at the NR2B subunit of the NMDA glutamate receptor; also has γ-aminobutyric acid (GABAA) receptor agonist properties.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
K D Laxer
The Western journal of medicine, 161(3), 309-314 (1994-09-01)
For the first time in 15 years, new antiepileptic medications are available for the treatment of patients with seizure disorders. These drugs have demonstrated efficacy in animal models of epilepsy and in controlled clinical trials. Felbamate was licensed in 1993
John M Pellock et al.
Epilepsy research, 71(2-3), 89-101 (2006-08-08)
An expert panel convened to evaluate data and review current clinical practices regarding the novel antiepileptic drug (AED) felbamate. Felbamate has demonstrated efficacy against a variety of refractory seizures types, including seizures associated with Lennox-Gastaut syndrome, but postmarketing experience revealed
Angelique M Leone et al.
Chemical research in toxicology, 20(4), 600-608 (2007-03-27)
Felbamate is an antiepileptic drug that is associated with minimal toxicity in preclinical species such as rat and dog but has an unacceptable incidence of serious idiosyncratic reactions in man. Idiosyncratic reactions account for over half of toxicity-related drug failures
N W Kleckner et al.
The Journal of pharmacology and experimental therapeutics, 289(2), 886-894 (1999-04-24)
Felbamate is an anticonvulsant used in the treatment of seizures associated with Lennox-Gastaut syndrome and complex partial seizures that are refractory to other medications. Its unique clinical profile is thought to be due to an interaction with N-methyl-D-aspartate (NMDA) receptors
J M Pellock
Epilepsia, 40 Suppl 5, S57-S62 (1999-10-26)
Felbamate (FBM) was the first of the new antiepileptic drugs (AEDs) approved in the United States in 1993 with broad-spectrum efficacy against partial and generalized seizures of various types, and indicated for use as adjunctive and monotherapy. The identification of
我們的科學家團隊在所有研究領域都有豐富的經驗,包括生命科學、材料科學、化學合成、色譜、分析等.
聯絡技術服務